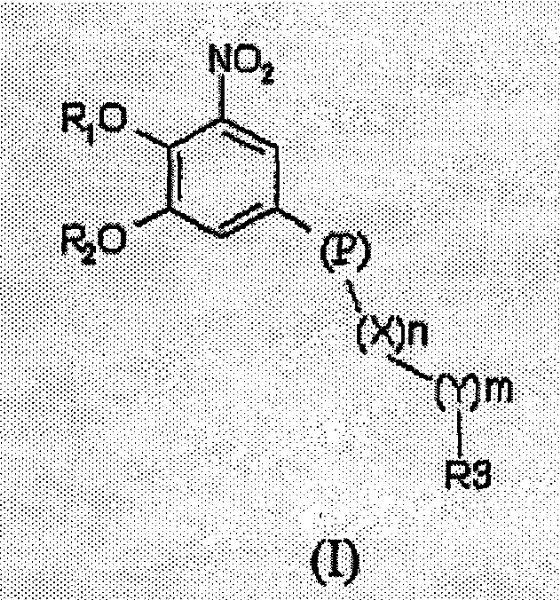
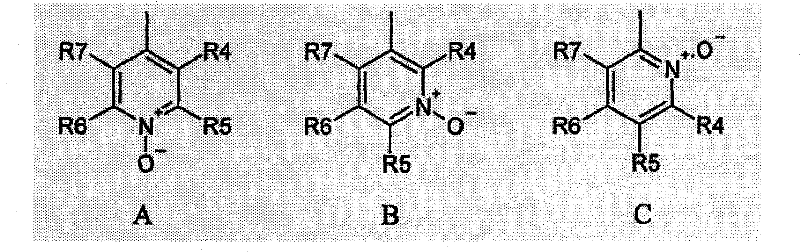
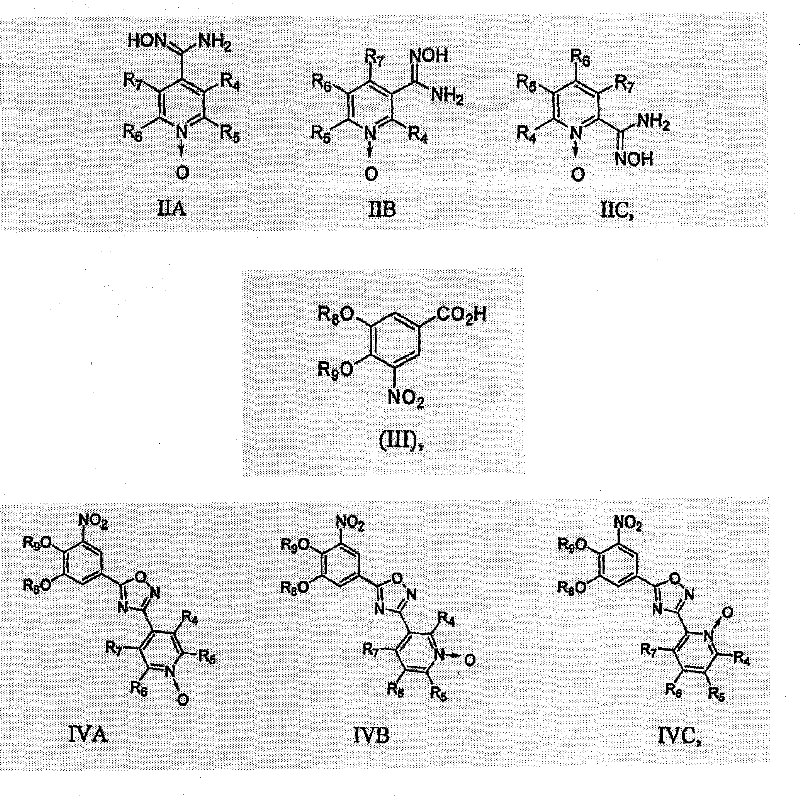
Nitrocatechol derivatives as COMT inhibitors
A nitro and nitrobenzene technology, applied in the field of novel substituted nitrocatechols, can solve the problem of low activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0118] 3-nitro-5-[3-(1-oxo-pyridin-4-yl)-[1,2,4]oxadiazol-5-yl]-benzene-1,2-diol (compound 4, Table 1)
[0119] a) To a stirred solution of 3,4-dibenzyloxy-5-nitrobenzoic acid (0.5 g, 1.32 mmol) in dimethylformamide (5 mL) was added 1 portion of 1 at room temperature, 1-Carbonyldiimidazole (0.246 g, 1.52 mmol). After stirring for 1 hour, 1 part of N'-hydroxypyridine-4-carboximidamide (0.208 g, 1.52 mmol) was added, and the resulting mixture was stirred at room temperature overnight. The mixture was then stirred at 110° C. for three hours and then allowed to cool to room temperature. The mixture was poured onto an ice-water bath (100 mL), and extracted with 20% isopropanol / dichloromethane. The organic extract was washed with water and brine, then dried (Na 2 SO 4 ), filtered and evaporated to leave a solid residue which was recrystallized from ethanol. 4-[5-(3,4-Dibenzyloxy-5-nitrophenyl)-[1,2,4]oxadiazol-3-yl]-pyridine was obtained as a beige solid (0.395 g, 62% ).
[...
Embodiment 2
[0123] 3-nitro-5-[3-(1-oxo-pyridin-3-yl)-[1,2,4]oxadiazol-5-yl]-benzene-1,2-diol (compound 5, Table 1)
[0124] a) To a stirred solution of 3,4-dimethoxy-5-nitrobenzoic acid (0.232 g, 1.022 mmol) in dimethylformamide (5 mL) was added 1 part of 1,1-carbonyl at room temperature Diimidazole (0.174 g, 1.073 mmol). The resulting mixture was stirred for ninety minutes, whereupon 1 part of N'-hydroxypyridine-3-carboximidamide 1-oxide (0.156 g, 1.022 mmol) was added. The resulting mixture was stirred at room temperature for two hours and then allowed to stand overnight at 75°C. After cooling to room temperature, the mixture was poured into water (100 mL), and the precipitate was filtered off, washed with water, then dried in air, and recrystallized from diethyl ether. 3-[5-(3,4-Dimethoxy-5-nitrophenyl)-[1,2,4]oxadiazol-3-yl]-pyridine 1-oxide (0.162 g, 46%).
[0125] b) Under argon protection, to a stirred solution of the above dimethyl ether (0.153 g, 0.445 mmol) in dichloromethane...
Embodiment 3
[0128] 3-nitro-5-[3-(1-oxo-pyridin-2-yl)-[1,2,4]oxadiazol-5-yl]-benzene-1,2-diol (compound 6, Table 1)
[0129] a) To a stirred solution of 3,4-dimethoxy-10-nitrobenzoic acid (1.0 g, 4.40 mmol) in dimethylformamide (10 mL) was added 1 part of 1,1-carbonyl at room temperature Diimidazole (0.821 g, 5.06 mmol). The resulting mixture was stirred for ninety minutes before adding 1 part of N'-hydroxypyridine-2-carboximidamide 1-oxide (0.775 g, 5.06 mmol). The resulting mixture was stirred overnight at room temperature, then poured into water (100 mL). The resulting precipitate was filtered off, washed with water, and added to dichloromethane (30 mL). The organic layer was washed with water and brine, dried (Na 2 SO 4 ), filtered, and evaporated to leave a white solid (1.37 g, 86%).
[0130] b) Under argon protection, to a stirred suspension of the above obtained solid (1.365 g, 3.77 mmol) in tetrahydrofuran (14 mL) was added a 1N solution of tetrabutylammonium fluoride in tetr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com