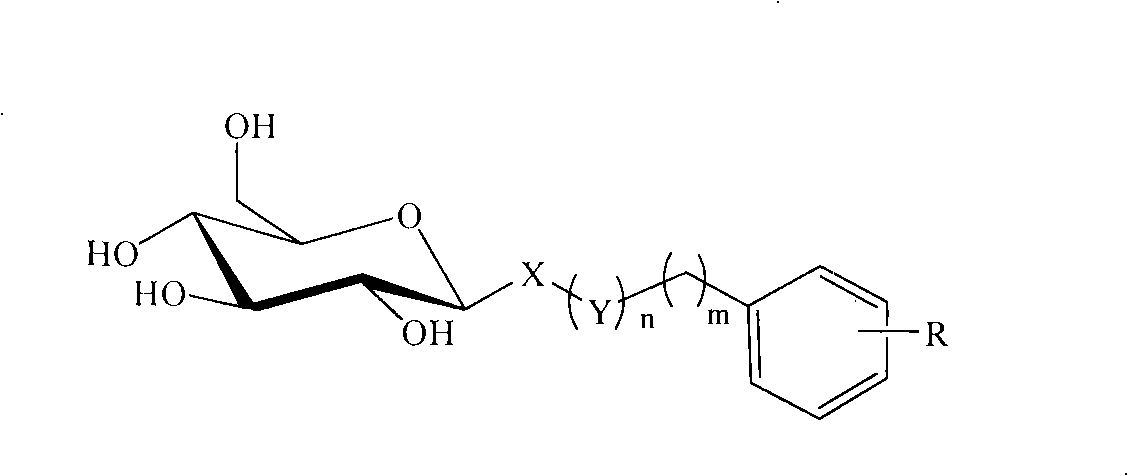
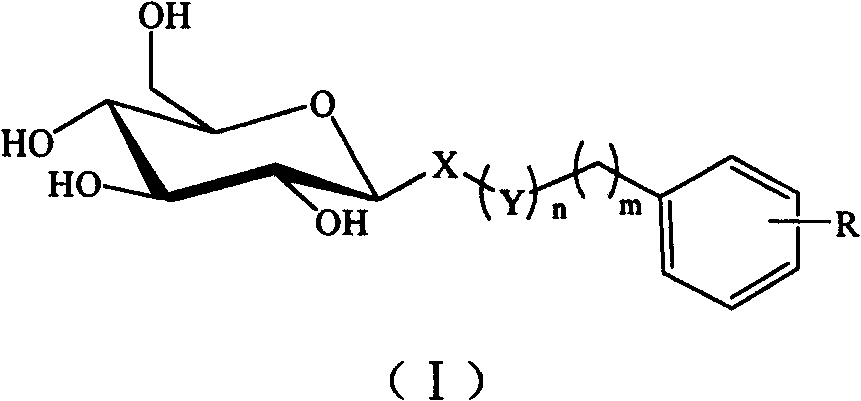
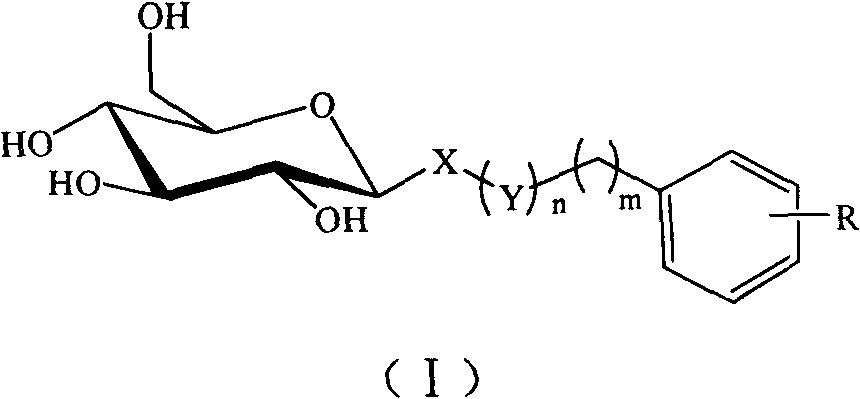
C-glycoside and C-glycoside compound containing substituted aromatic rings as well as preparation and use thereofb
A technology of compound and sugar carbon glycosides, which is applied in the field of medicine, can solve the problem of nerve damage without therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: (3',4',5'-trimethoxyphenyl)methyl-1-thio-β-D-glucopyranoside (S01)
[0030] A: (3′,4′,5′-trimethoxyphenyl)methyl-2,3,4,6-tetra-O-acetyl-1-thio-β-D-glucopyranoside
[0031] 1.31g (5mmol) 1-bromomethyl-3,4,5-trimethoxybenzene and 1.82g (5mmol) 2,3,4,6-tetra-O-acetyl-β-D-1-sulfur Dissolve glucopyranose in 30mL of acetone, add 0.69g (5mmol) of anhydrous potassium carbonate, and heat to reflux for 6h. After cooling, filter, wash the solid with acetone, and concentrate the filtrate to remove the solvent to obtain 2.70 g of syrup, and the yield of the crude product is 99.2%. R f =0.25, petroleum ether (60-90°C):ethyl acetate=2:1.
[0032] B: (3′,4′,5′-trimethoxyphenyl)methyl-1-thio-β-D-glucopyranoside (S01)
[0033] 2.70g (4.96mmol) of (3',4',5'-trimethoxyphenyl)methyl-2,3,4,6-tetra-O-acetyl-1-thio-β-D- Glucopyranoside was dissolved in 30 mL of methanol, 4.14 g (30 mmol) of anhydrous potassium carbonate was added, and the reaction was stirred at room temperatur...
Embodiment 2
[0034] Example 2: (4'-methylphenyl)methyl-1-thio-β-D-glucopyranoside (S02)
[0035] A: (4′-methylphenyl)methyl-2,3,4,6-tetra-O-acetyl-1-thio-β-D-glucopyranoside
reference example 1
[0036] Reference Example 1 Step A reaction synthesis, to obtain 2.20g syrup, crude product yield 94.0%. R f =0.71, petroleum ether (60-90°C):ethyl acetate=2:1.
[0037] B: (4′-methylphenyl)methyl-1-thio-β-D-glucopyranoside (S02)
[0038] Reference Example 1 Step B reaction synthesis, prepared 1.17g syrup, yield 83.0%. 1 HNMR (CD 3 OD) δ (ppm): 2.29 (3H, s, -CH 3 ), 3.17-3.31 (4H, H-5, H-4, H-3, H-2), 3.65 (1H, dd, H-6a, J 1 =6.2Hz,J 2 = 12.2Hz), 3.79 (1H, d, -CHPh', J = 13.2Hz), 3.88 (1H, dd, H-6b, J 1 =2.2Hz,J 2= 12.2Hz), 3.99 (1H, d, -CHPh', J = 13Hz), 4.13 (1H, d, H-1, J = 9.6Hz), 7.10 (2H, d, Ph', J = 7.8Hz) , 7.22 (2H, d, Ph', J = 8.1 Hz). -c ESI m / z: 299(M-1), 359(M+Na+2H 2 O), 599(2M-1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com