Diheterocycle compound containing 4-thiazolidinone and pyrimidine, synthetic method and application thereof
A kind of technology of thiazolidinone and synthesis method, applied in the field of 4-thiazolidinone and pyrimidine bi-heterocyclic compound and synthesis thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
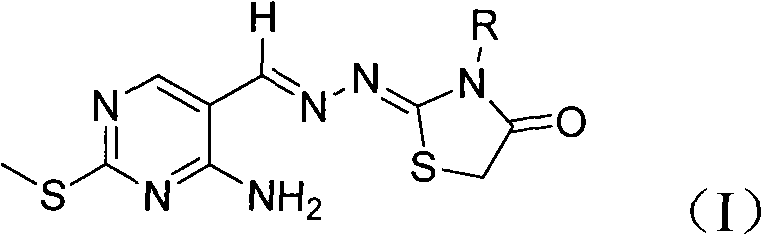
[0030] Example 1: Preparation of 2-[2-[(2-methylthio-4-amino-5-pyrimidine)methylene]hydrazone]-3-methyl-4-thiazolidinone
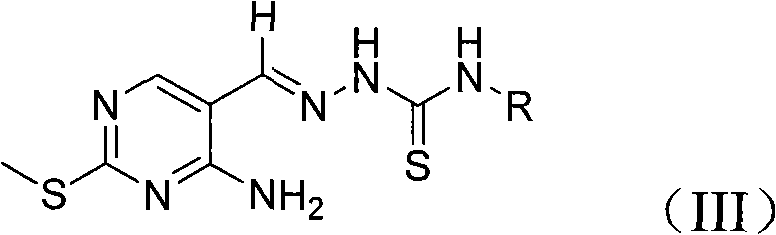
[0031] Add 0.34g (2mmol) 4-amino-2-methylthio-pyrimidine-5-aldehyde, 0.25g (2.4mmol) 4-methyl-thiosemicarbazide, 20mL ethanol and 0.42g acetic acid into a 50mL flask, The reaction was refluxed for 12 hours under stirring, and the reaction was completed. The reaction solution was concentrated to about 10 mL, cooled to room temperature, and the product was precipitated, filtered, and the product was washed with ethanol to obtain 0.44 g of white powder 1-[(2-methylthio-4- Amino-5-pyrimidine)methylene]-4-methyl-thiosemicarbazide. The yield was 86%.
[0032] 0.26g (1mmol) 1-[(2-methylthio-4-amino-5-pyrimidine) methylene]-4-methyl-thiosemicarbazide, 0.20g (1.2mmol) ethyl bromoacetate, 0.10 Add g (1.2mmol) anhydrous sodium acetate and 20mL ethanol into a 50mL flask, reflux for 10h under stirring, the reaction is over, concentrate the reaction solution to about ...
Embodiment 2
[0038] Example 2: Preparation of 2-[2-[(2-methylthio-4-amino-5-pyrimidine)methylene]hydrazone]-3-methyl-4-thiazolidinone
[0039] Get 0.26g (1mmol) 1-[(2-methylthio-4-amino-5-pyrimidine) methylene]-4-methyl-thiosemicarbazide prepared by the method of Example 1, cyclization reagent Change to 0.17g (1.2mmol) bromoacetic acid, other operations are the same as in Example 1. 2-[2-[(2-methylthio-4-amino-5-pyrimidine)methylene]hydrazone]-3-methyl - The yield of 4-thiazolidinone was 63%.
Embodiment 3
[0040] Example 3: Preparation of 1-[(2-methylthio-4-amino-5-pyrimidine) methylene]-4-methyl-thiosemicarbazide
[0041] The reaction solvent was changed to 20mL toluene, and other operations were the same as in Example 1. The yield of 1-[(2-methylthio-4-amino-5-pyrimidine) methylene]-4-methyl-thiosemicarbazide was 80% %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com