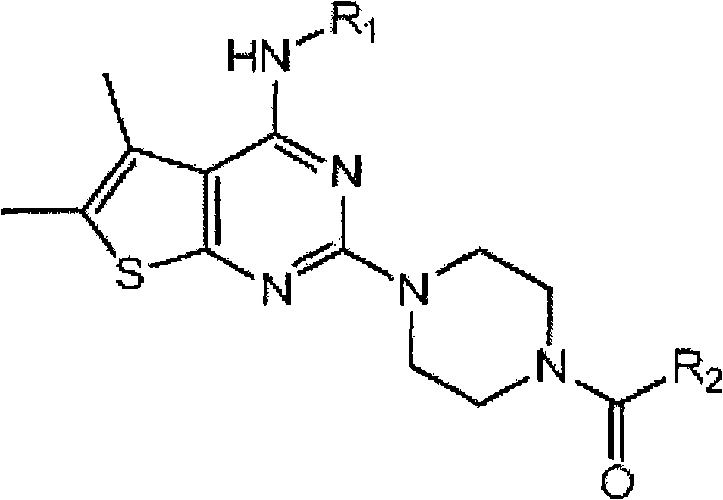
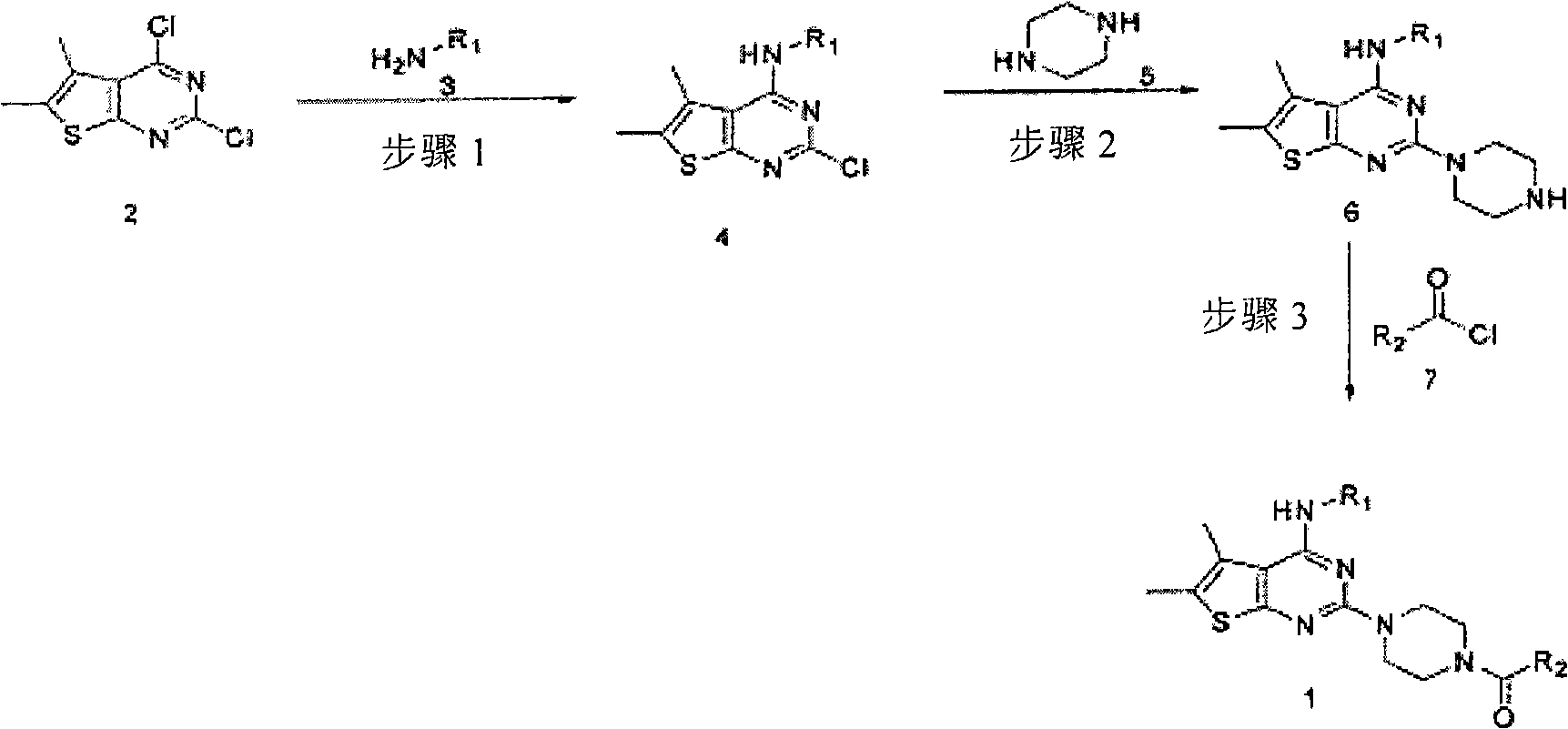
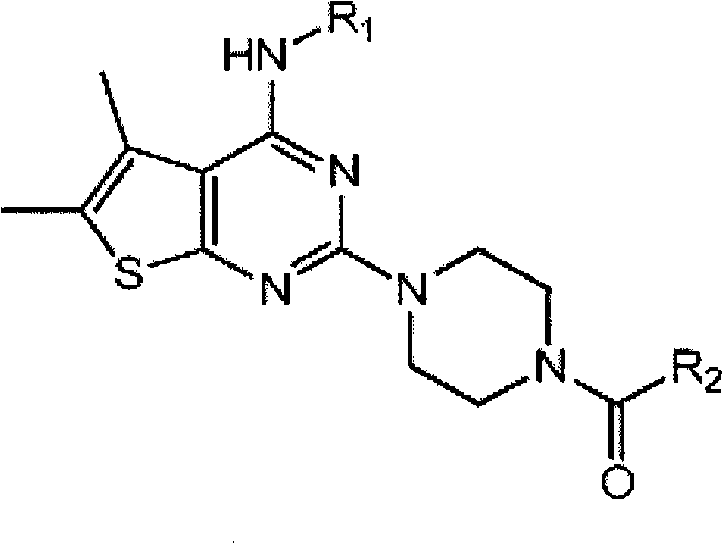
5,6-dimethylthieno(2,3-DI) pyrimidine derivatives, the preparation method thereof and the pharmaceutical composition comprising the same for anti-virus
A technology of dimethylthiophene and pyrimidine derivatives, which is applied in drug combinations, antiviral agents, digestive system, etc., and can solve the problems of ineffective therapeutic methods, no effective drugs for HCV development, and low cure rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: 4-[5,6-Dimethyl-4-(4-morpholin-4-yl-phenylamino)-thieno[2,3-d]pyrimidine Preparation of -2-yl]-piperazin-1-yl-pyridin-2-yl-methanone
[0039]220.20 mg (1.23 mmol) of 4-(4- Morpholino) aniline and 130.90 mg (1.23 mmol) of aniline, and then the mixture was stirred under reflux. After the reaction was complete, the reaction solvent ethanol was evaporated under reduced pressure, and the residue was crystallized from ethanol and filtered to yield 430 mg (quantitative) 2-chloro-5,6-dimethyl-thieno[2,3-d ] pyrimidin-4-yl-4-morpholin-4-yl-aniline. 220 mg (0.53 mmol) of the obtained product was dissolved in 20 ml of 1-butanol in a sealed tube, and 223 μl (1.59 mmol) of triethylamine and 45.9 mg of piperazine were added thereto, and the mixture was stirred at 120° C. . After the reaction was complete, the resulting solid was filtered and the filtrate was concentrated under reduced pressure, then crystallized from ethyl acetate to yield 30 mg (15% yield) of 5,6-di...
Embodiment 2
[0041] Example 2: 4-[5,6-Dimethyl-4-(4-morpholin-4-yl-phenylamino)-thieno[2,3-d]pyrimidine-2- Base]-piperazin-1-yl-pyridin-3-yl-methanone
[0042] The title product (45% yield) was obtained in the same manner as in Example 1 except that nicotinoyl chloride hydrochloride was used instead of picolinoyl hydrochloride.
[0043] 1 H NMR (CDCl 3 )ppm: 9.20 (s, 1H), 8.99-9.05 (m, 1H), 8.88-8.99 (m, 1H), 8.11-8.30 (m, 1H), 7.25-7.38 (d, 2H), 6.85-6.95 ( d, 2H), 3.92-3.76 (m, 10H), 3.42 (br, 2H), 3.18-3.14 (t, J=3.4Hz, 4H), 2.53 (s, 3H), 2.39 (s, 3H).
Embodiment 3
[0044] Example 3: 4-[5,6-Dimethyl-4-(4-morpholin-4-yl-phenylamino)-thieno[2,3-d]pyrimidine-2- Base]-piperazin-1-yl-pyridin-4-yl-methanone
[0045] The title product (48% yield) was obtained in the same manner as in Example 1 except that isonicotinoyl chloride hydrochloride was used instead of picolinoyl hydrochloride.
[0046] 1 H NMR (CDCl 3 )ppm: 8.75(d, J=5.6Hz, 2H), 7.46(d, J=9.0Hz, 2H), 7.34(d, J=1.6Hz, 2H), 7.04(s, 1H), 6.93(d, J=8.8Hz, 2H), 3.92-3.76(m, 10H), 3.41(br, 2H), 3.19-3.14(t, J=3.4Hz, 4H), 2.51(s, 3H), 2.39(s, 3H )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com