A method for quantification of allergens
An allergenic, absolute quantity technique, applied in the direction of measuring devices, markers used in chemical analysis, instruments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
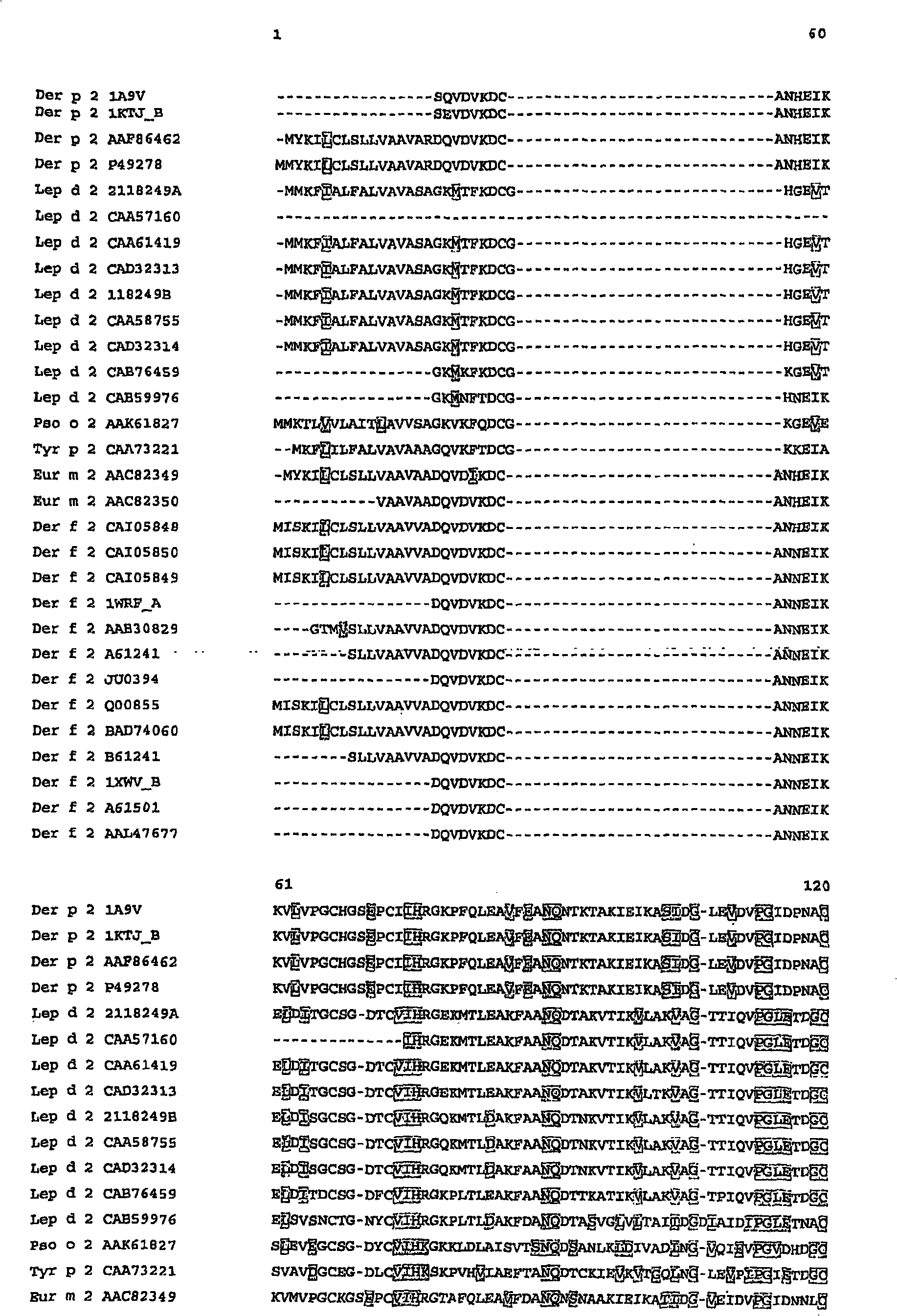
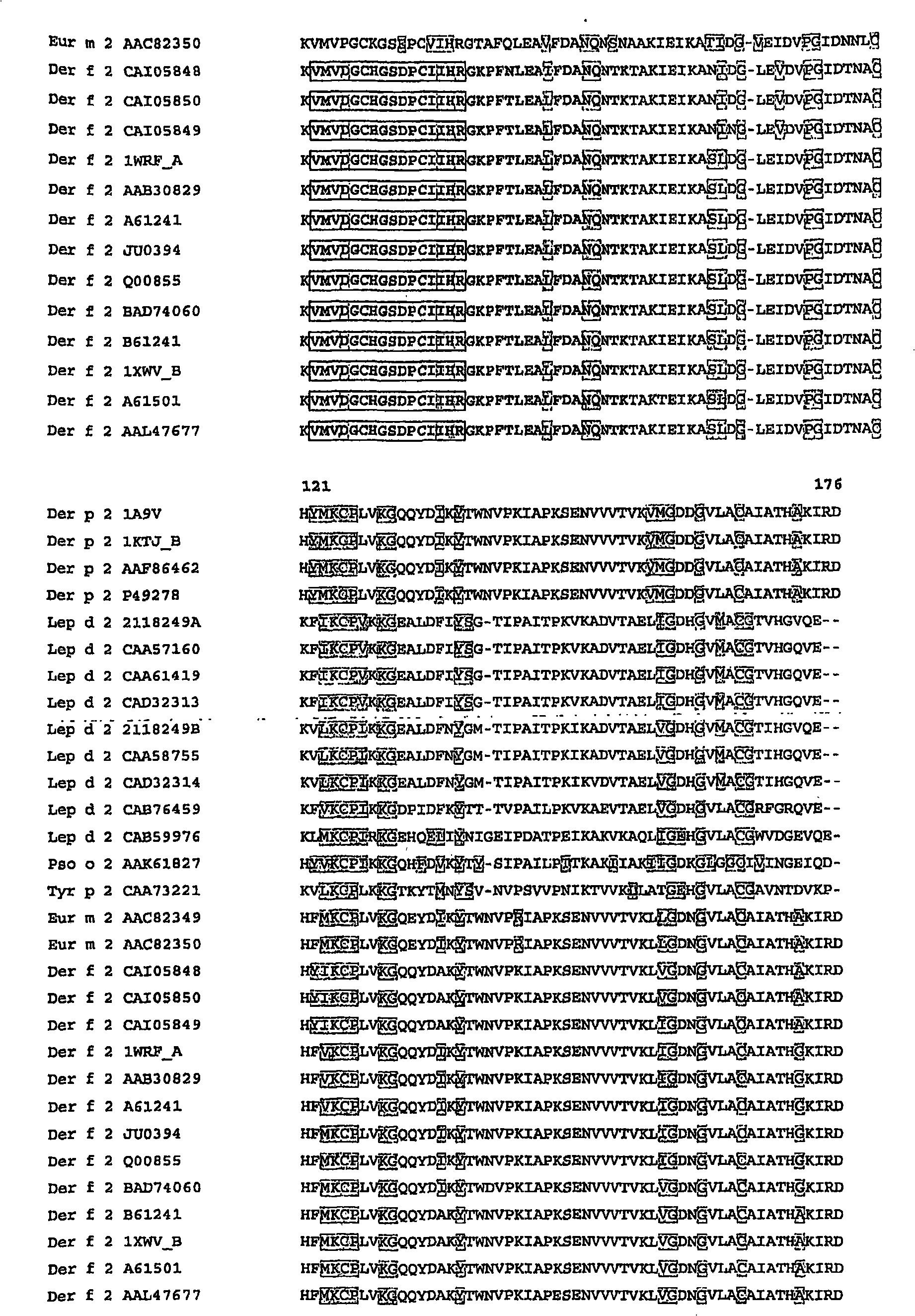
[0147] Identification of unique constant regions for Der f2, Der p2, Phl p1, Phl p5, and Bet v1 along with synthetic calibration standard peptides
[0148] Absolute quantification of allergens using unique constant region sequences, ie marker peptides in native Der f2, Der p2, Phl p1, Phl p5 and Bet v1, can be demonstrated using two different means. Synthesis of two calibration standard peptides for iTRAQ TM Labeling technology (Applied Biosystems) was evaluated. In addition, four calibration standard peptides were synthesized to evaluate Protein-AQUA TM (Sigma-Aldrich) and other isotope labeling techniques.
[0149] Based on the amino acid sequence alignment (Vector NTI) (Figure 1), the cleavage analysis carried out by GPMAW ( figure 2 ) and Blast database search to design allergen calibration standard peptides with corresponding to 32-48 in Der f2 and Der p2, 149-158 in Phl p1, 123-135 in Phl p 5a, Phl p 5b Species-specific sequences of the amino acid sequences 115-127 ...
Embodiment 2
[0152] Purified natural and recombinant grass, birch and mite group 2 allergens
[0153]Native Der f2 and Der p2 were purified from 100 mg of Dermatophex farinae and house dust mite extracts (ALK-Abelló, Horsholm, Denmark). Native Phl p 1 and 5 were purified from 50 mg Phleum prantense extract (ALK-Abelló) and native Bet v1 was purified from 50 mg Betulaverrucosa extract (ALK-Abelló). Purification of the molecules was performed as described (Johannessen BR et al. FEBS Lett 2005; 579: 1208-12, Aasmul-Olsen S. et al. New Horizons in Allergy Immunotherapy, edit by Sehon et al. Plenum Press. New York 1996, p.261-65, Petersen A et al. Clin Exp Allergy. 1994 Mar;24(3):250-6, Ipsen H & Lowenstein H J Allergy Clin Immunol. 1983;72(2):150-59). Recombinant Der f2 and Der p2 were expressed in the Pichia pastoris expression system and purified as described (Johannessen BR et al. FEBS Lett 2005;579:1208-12). Proteins were stored at -20°C as lyophilized aliquots.
[0154] Using one of th...
Embodiment 3
[0156] Pre-fractionation with HDM extract and protein digestion
[0157] Pre-fractionation of dust mite (Der f) and house dust mite (Der p) extracts by hydrophobic interaction chromatography. Fractionation of mite extracts was performed with a 1.0 ml HiTrap Phenyl column (GE-Healthcare, Uppsala, Sweden). Equilibrate the column with 50 mM sodium phosphate buffer (Merck, Darmstadt, Germany), pH 7.0, 1.0 M ammonium sulfate (Fluka, Buchs, Switzerland), buffered with 5 column volumes of 50 mM sodium phosphate with a decreasing linear gradient. solution (Merck), pH 7.0 to elute the bound sample. Chromatography was performed separately for each of the HDM extracts (Der f and Der p). Based on the analysis of the fractions by SDS-PAGE (Invitrogen, Carlsbad, CA, USA), the HDM proteins were divided into two major protein pools ( Figure 4 ). Der f and Der p pools containing HDM 2 allergen were dialyzed against 10 mM ammonium bicarbonate (BDH, Poole, England). The dialyzed Der f and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com