Pharmaceutical composition for treating large intestinal cancer
A composition and colorectal cancer technology, applied in the chemical field, can solve problems such as unsatisfactory effects, and achieve the effect of inhibiting the growth of human colorectal cancer cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Cell culture
[0050] The medium of SW480 cells (purchased from the Cell Resource Center of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, SIBC) is RPMI-1640 complete culture medium (10% fetal bovine serum, 100U / ml penicillin / streptomycin), HT29 and HCT116 cells ( The medium purchased from the Cell Resource Center, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, SIBC) is McCOY'S 5A MEDIUM (Sigma, USA) complete culture medium (10% fetal bovine serum, 100U / ml penicillin / streptomycin), the three Culture conditions are 95% air, 5% CO 2 , 95% humidity, 37℃ constant temperature.
Embodiment 2
[0051] Example 2 MTT colorimetric experiment
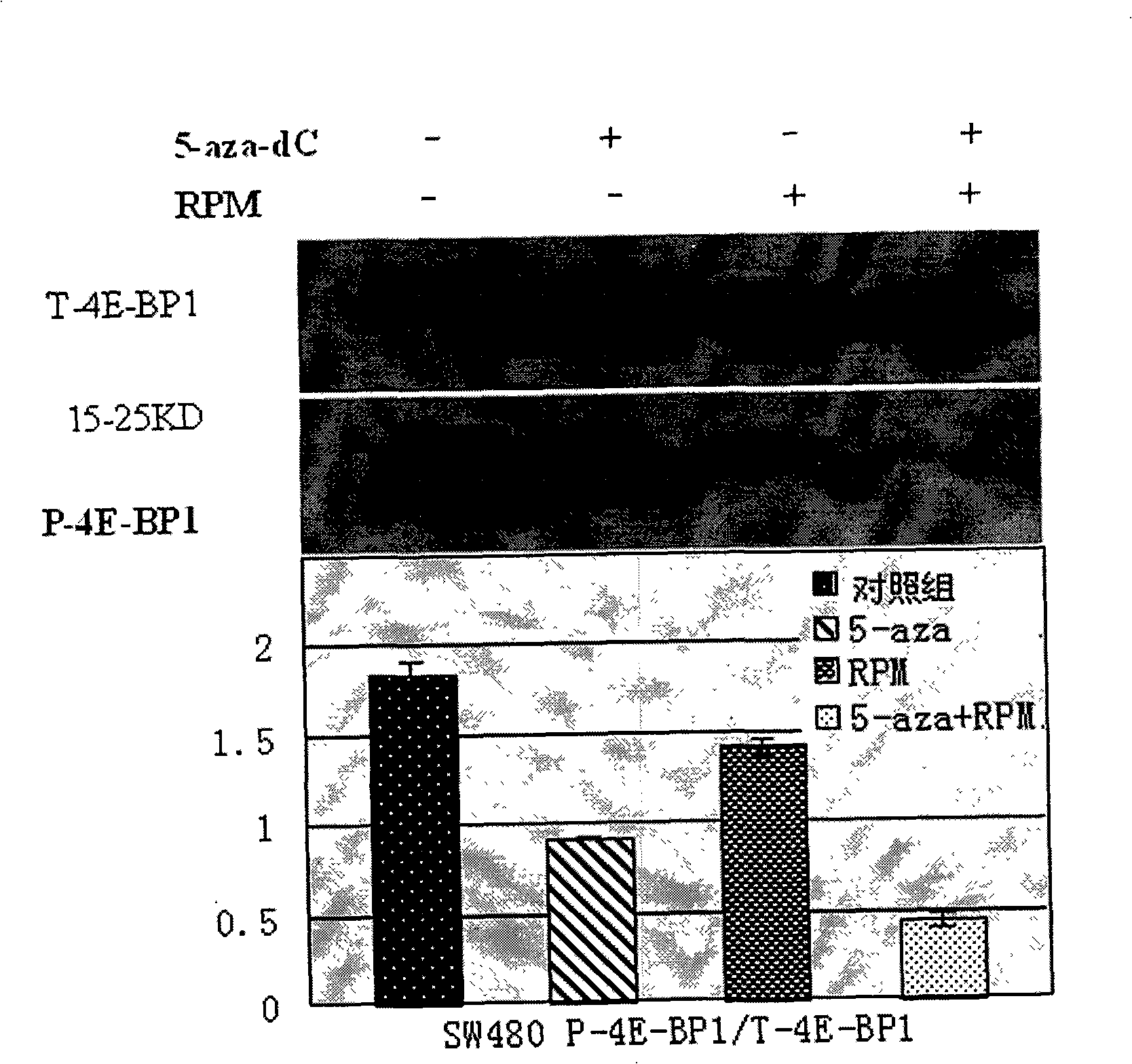
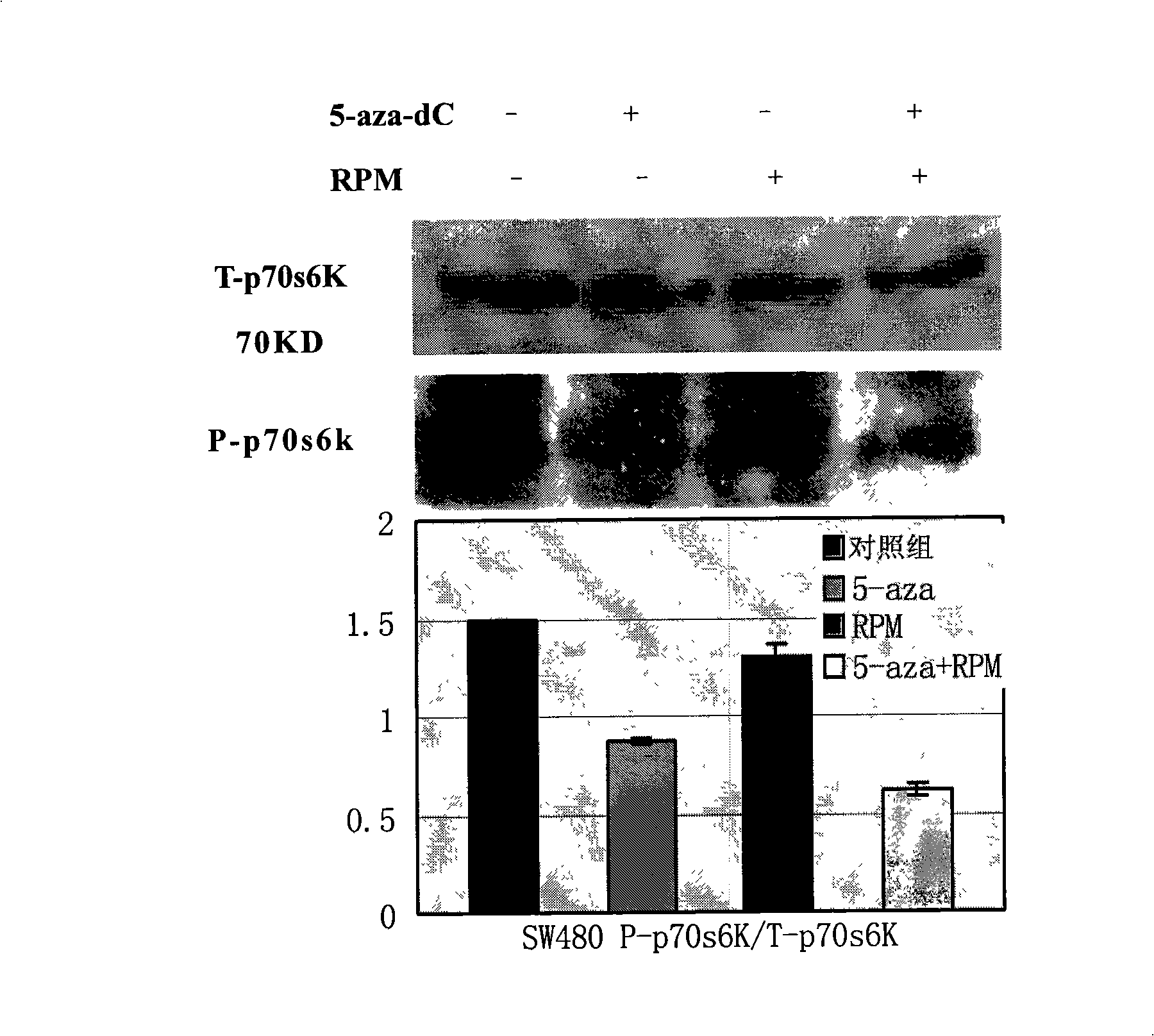
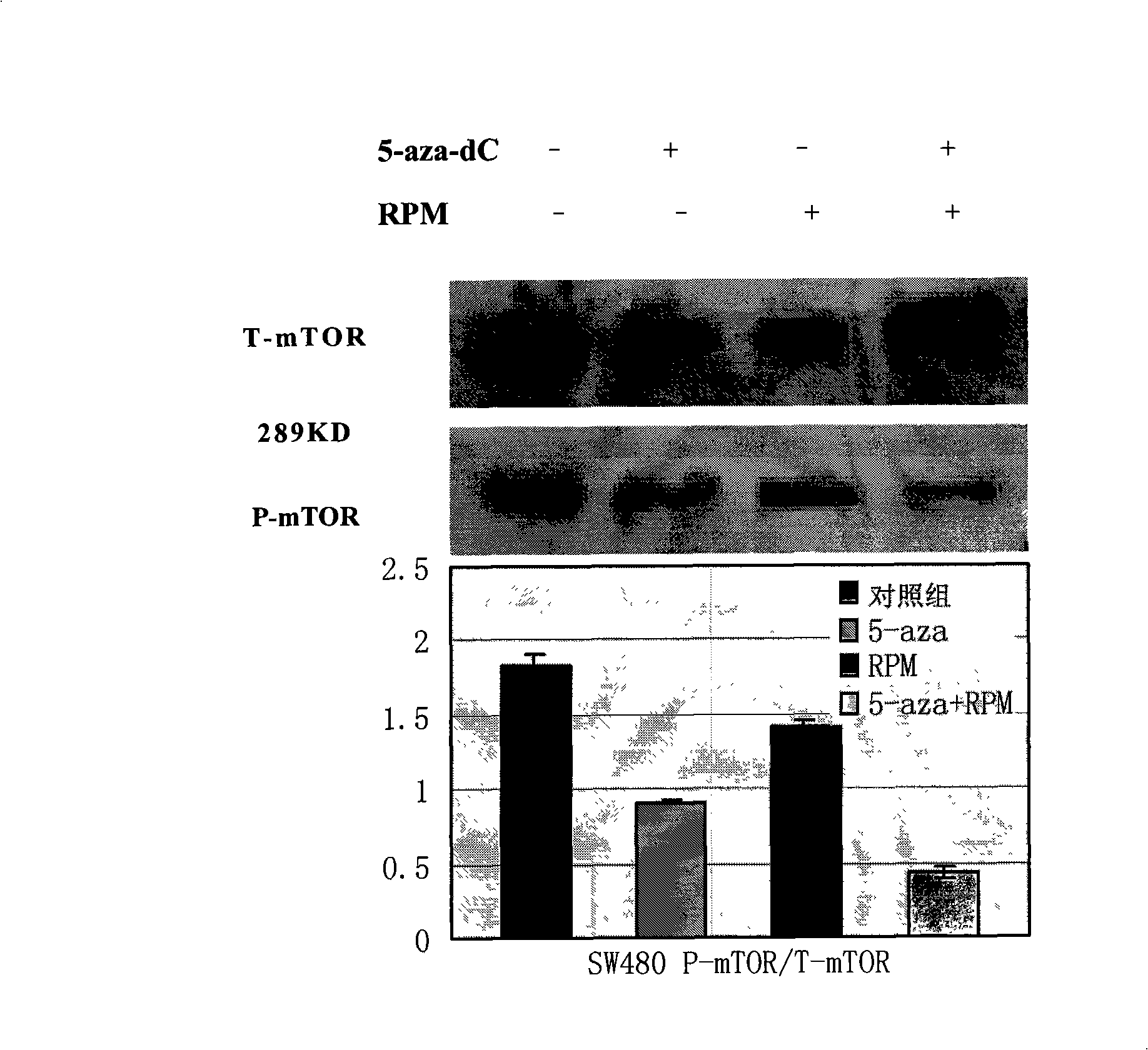
[0052] Dilute the single cell suspension to 2×10 in complete culture medium 4 / ml~3×10 4 / ml, inoculate to 96-well culture plate at 100μl / well. After culturing for 24 hours, when the cells are in the logarithmic growth phase, discard the original culture medium and add each group of intervention drug working solutions (set the following drug intervention groups: 5-aza-dC 5μmol / L, 5-aza-dC 10μmol / L , RPM 10nmol / L, 5-aza-dC 5μmol / L+RPM 10nmol / L, 5-aza-dC 10μmol / L+RPM 10nmol / L; combined action in 5-aza-dC 5μmol / L+RPM 10nmol / L Group, the mass ratio of 5-aza-dC to RPM is 124.8:1, in the 5-aza-dC 10μmol / L+RPM 10nmol / L combined action group, the mass ratio of 5-aza-dC to RPM is 249.6:1), The control group was added with complete culture medium, 0.5% PBS, 0.5% dimethyl sulfoxide (DMSO). After continuing to incubate for 24, 48, 72, 96 hours, add 5 mg / ml MTT (methyl thiazolyl bluetetrazolium bromide, (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenytetra...
Embodiment 3
[0058] Example 3 Extraction of total cell protein
[0059] Following Example 2, 5-aza-dC 10μmol / L, RPM 10nmol / L, RPM10nmol / L+5-aza-dC 10μmol / L were treated for 48h, and then the total cell protein was extracted by RIPA lysis method. The specific steps are as follows: Cells Lysis buffer (Tris.HCl, 50mmol / L, pH 7.5; NaCl, 150mmol / L; NP-40, 1%; Sodium deoxycholate, 0.5%; SDS, 0.1%; EDTA, 1mmol / L; PMSF, 1mmol / L; Leupeptin, 2μg / ml) 100μl suspended cell cluster; vortex for 30s, stand on ice for 5min, repeat three times; centrifuge at 14000rpm 4℃ for 10min, collect the supernatant, which is the total protein.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com