Cryopreservation of hepatocytes
A technology of cryopreservation and hepatocytes, applied in the preservation of human or animal bodies, animal cells, vertebrate cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Preparation of human hepatocytes for cryopreservation
[0040] 1.1 Isolation of human hepatocytes
[0041] Hepatocytes from human donors are isolated in a manner known per se from tissue parts surgically removed by any means, which requires the consent of the donor. To this end, the tissue is perfused, the hepatocytes are detached from the tissue complex, and the hepatocytes can be obtained from the perfusion solution. The viability of harvested hepatocytes was determined by trypan blue staining. For further experiments, only cell preparations showing trypan blue exclusion above 70% were used.
[0042] 1.2 Preparation of matrix
[0043] In the following experiments, cell culture vessels in the form of multiwell plates, 6-well plates (type 657160, GreinerBio-One) were used. Culture plates are coated with native collagen gel, preferably isolated from rat tails. Alternatively, multiwell plates were used - 6-well plates pre-coated with type I collagen (typ...
Embodiment 2
[0054] Example 2: Thawing of Cryopreserved Hepatocytes
[0055] Hepatocyte cultures frozen according to Example 1 and stored at -151°C were thawed and re-cultured after a storage period of up to 4 weeks for further use in in vitro experiments.
[0056] For thawing of cryopreserved hepatic parenchymal cell cultures, the 6-well plates were first immediately transferred to an incubator operating under standard conditions for 5 minutes after removal from the freezer or nitrogen tank (see Example 1). Subsequently, 1 ml of solution 1 preheated to 37° C. (see Example 1) was slowly added dropwise to each well. This method was repeated for up to 3 simultaneously thawed 6-well plates, ie up to 18 wells.
[0057] Subsequently, the process was repeated, and 1 ml of solution 1 was slowly added to each well. Then, aspirate the supernatant with a Pasteur pipette. The supernatant essentially contained thawed non-viable and non-adherent cells.
[0058] For further washing, in each case, ...
Embodiment 3
[0062] Embodiment 3: the mensuration of living cell number
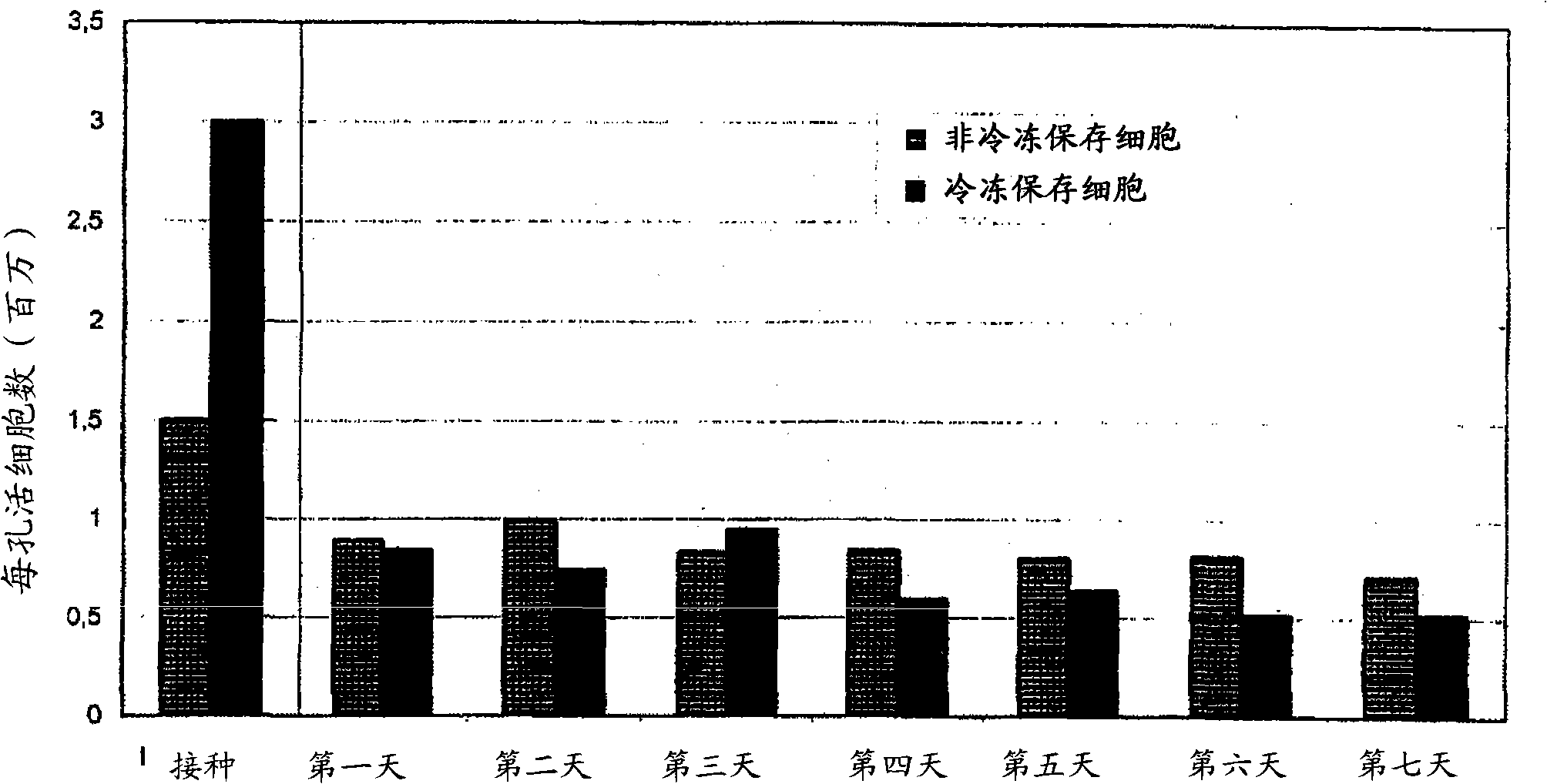
[0063] The number of viable (living) cells in cultures of freshly isolated hepatocytes and in cultures of cryopreserved hepatocytes according to the invention was determined by morphologically intact cell counting methods. Here, for 0.259mm 2 Photos of the size of the control area were counted. From the number of intact cells found in the control area, the number of intact cells in the entire cell culture vessel (for the 9.6 cm well used 2 area of a 6-well plate, the correction factor is 3700).
[0064] Morphology of re-cultured hepatocytes was photographically documented before freezing and at various time points after thawing from cryopreservation and compared with cultures of freshly isolated and cultured hepatocytes.
[0065] result:
[0066] Figure 1 shows the morphology of human hepatocytes freshly isolated and seeded on the collagen gel layer ( Figure 1A ) and the morphology of isolated human hepatocyte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com