Inhibitors of inflammatory cytokine transcription derived from hcmv protein IE2
A technology of human cytomegalovirus and cytomegalovirus, which is applied in the treatment of inflammation and inflammatory diseases, and can solve the problems of side effects and high production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
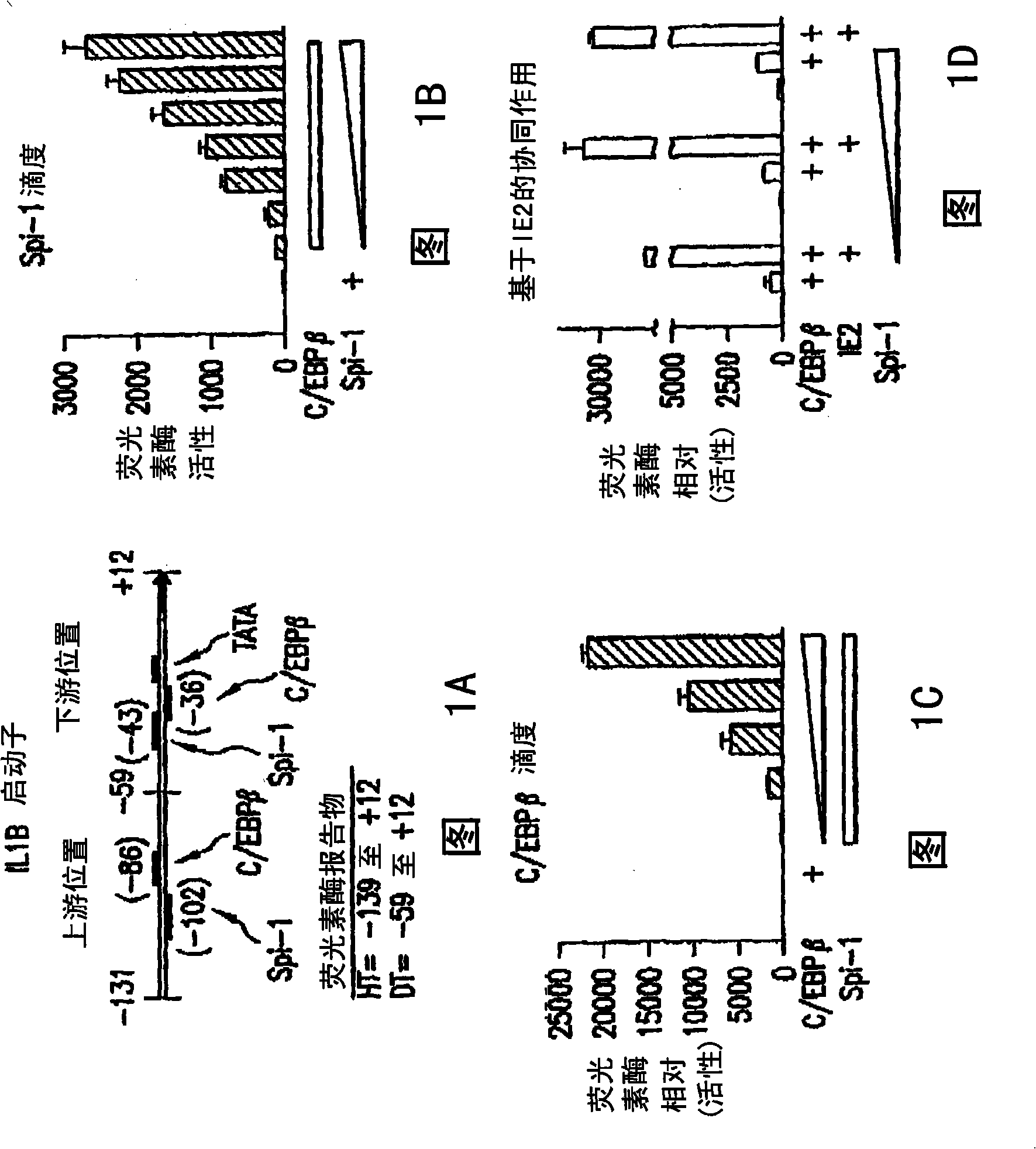
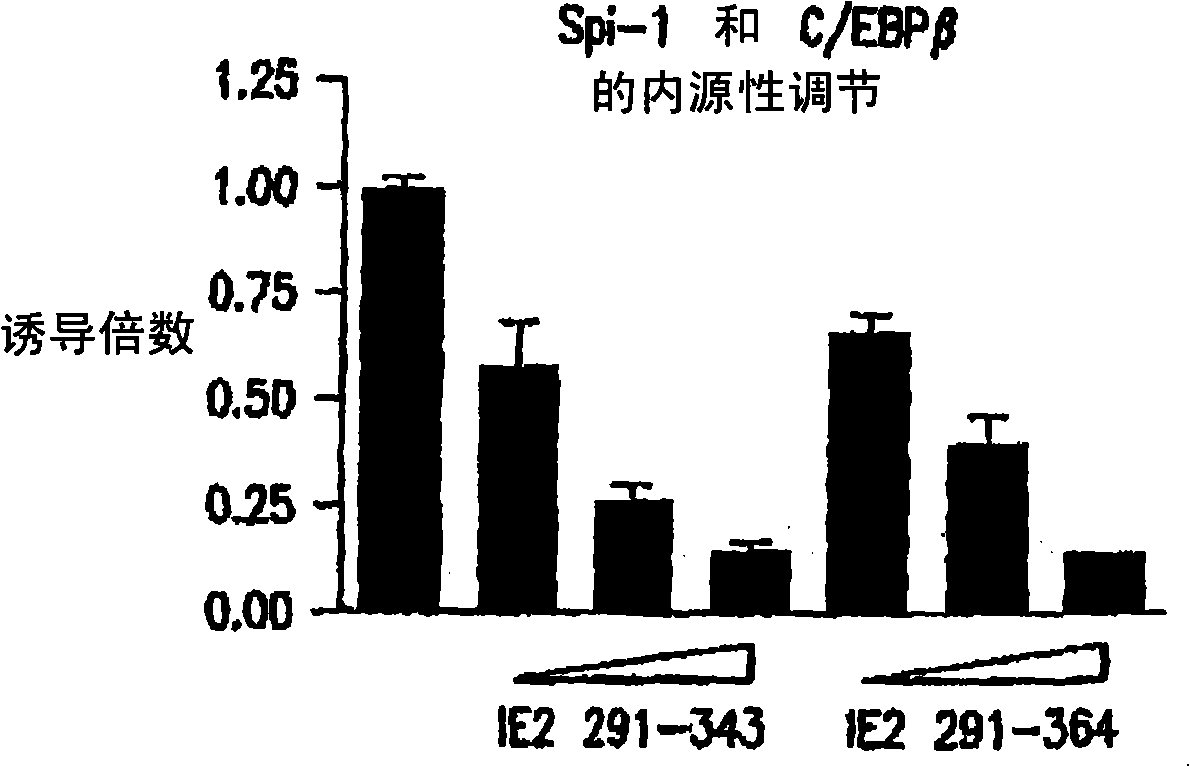
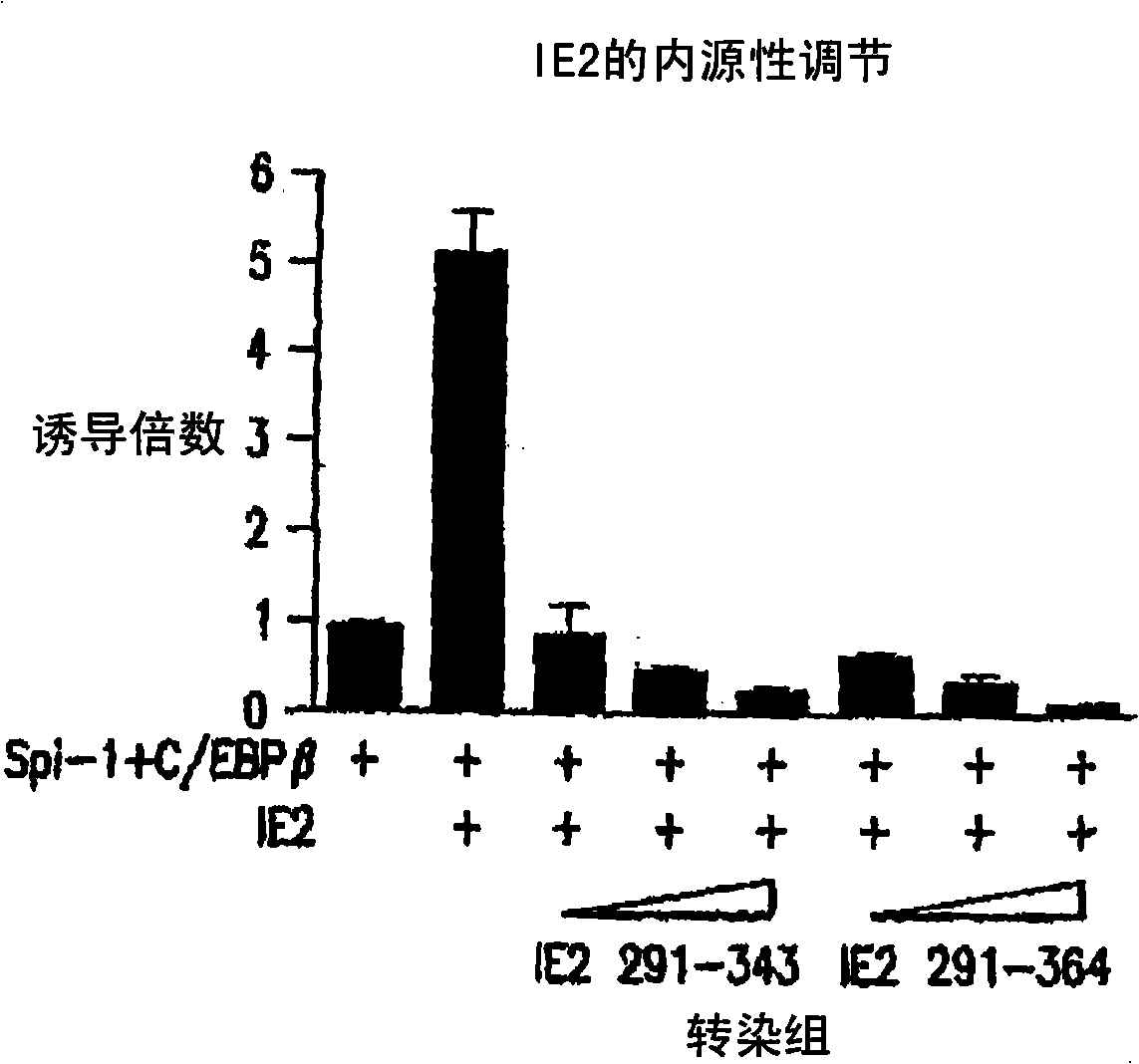
[0100] IE2 fragments inhibit Spi-1 function on IL-1β
[0101] The functional inhibition of Spi-1 on IL-1β promoter by IE2 291-364 was detected by gene reporter assay. In this system, the IL-1β promoter is cut in front of the firefly luciferase reporter gene. When this promoter is activated by Spi-1, the cells will produce firefly luciferase. This enzyme activity is measured by how the cells light up. This general technique was employed because it is an easier and more sensitive method of measuring promoter function than gene products such as IL-1[beta].
[0102] When Spi-1 and C / EBPβ were transfected into HeLa-S3 cells, the reporter showed a titratable response (Fig. 1B and C). However, when full-length wild-type IE2 was added to the transfection of cells, the activity of the gene was increased by 4-10 fold (Fig. 1D). This demonstrates the efficacy of IE2. However, if the inhibitor peptide was added dropwise, the activity of the reporter was reduced both in the absence ...
Embodiment 2
[0106] IE2 Fragment Expression in Transfection Assay
[0107] In order to verify the IE2 fragments expressed in the transfection experiments, these fragments were inserted into the GFP expression vector to detect whether they were localized in the nucleus ( Figure 4A ). The results indicate the expression of the protein and the function of the putative nuclear localization sequence located in the IE2 fragment.
[0108] 24 hours after transfection, GFP 291-364 fragments were found in both cytoplasm and nucleus. The GFP 291-364 fragment inhibits the endogenous transactivation of the HT reporter gene by Spi-1 and C / EBPβ. To control transfection efficiency, GFP-expressing cells were first purified by FACS. Equal numbers of cells were then used to prepare lysates for reporter assays. These data show that both GFP fusion products retain the dominant negative function ( Figure 4B ). This function is specific to the inserted IE2 fragment, since the reporter gene activity was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com