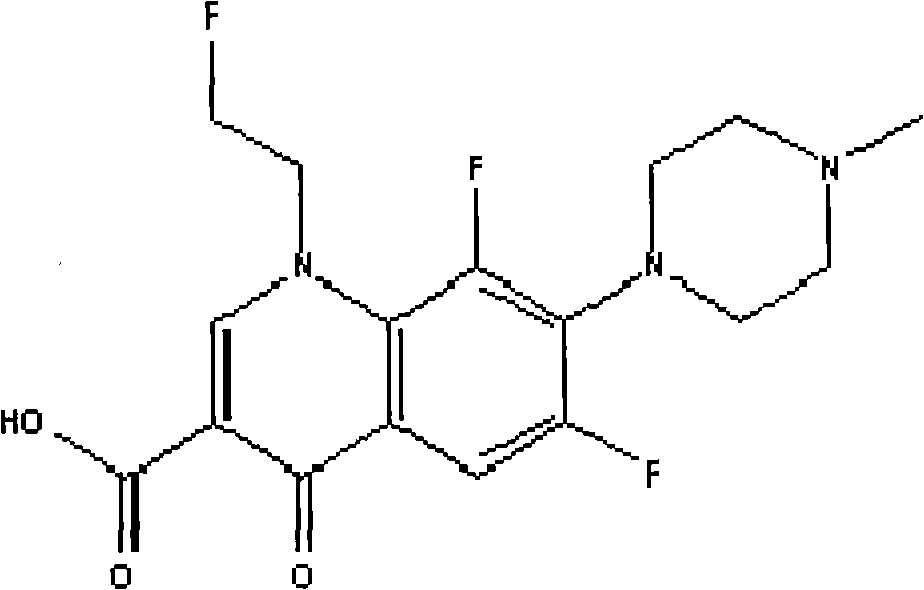
Fleraxacin for injection and preparation method thereof
A fleroxacin and injection technology, which is applied in the field of fleroxacin freeze-dried powder for injection and its preparation field, can solve the problems of inconvenience in injection use, turbidity, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] prescription:
[0044] Fleroxacin 206.08g
[0045] Mannitol 100g
[0046] Lactic acid 85.42ml
[0047] Add water for injection to 2000ml
[0048]
[0049] Total 1000 bottles
[0050] Preparation process: Add 206.08g of fleroxacin and 100g of mannitol into 1600mL of water for injection, stir, then dropwise add 85.42mL of lactic acid to the above mixture, stir until fleroxacin is completely dissolved, then add 0.6g of Activated carbon, stirred at room temperature for 30 minutes, filtered through a 0.45 μm microporous membrane to remove the activated carbon, then added water for injection to 2000 mL, stirred thoroughly for 20 minutes to mix the solution evenly, and the obtained mixture was sterilized by filtering through a 0.22 μm microporous membrane, and the filtrate was placed in Slowly cool to -30°C in the tray of the freeze dryer, and maintain -30°C for 3 hours, then quickly cool to -55°C, vacuumize, heat up to 2°C within 14 hours, and...
Embodiment 2
[0052] prescription:
[0053] Fleroxacin 206.08g
[0054] Mannitol 82.5g
[0055] Lactic acid 80.31ml
[0056] Add water for injection to 2000ml
[0057]
[0058] Total 1000 bottles
[0059] Preparation process: Add 206.08g of fleroxacin and 82.5g of mannitol into 1600mL of water for injection, stir, then dropwise add 80.31mL of lactic acid to the above mixture, stir until fleroxacin is completely dissolved, then add 0.68g of Activated carbon, stirred at room temperature for 30 minutes, filtered through a 0.45 μm microporous membrane to remove the activated carbon, then added water for injection to 2000 mL, stirred thoroughly for 20 minutes to mix the solution evenly, and the obtained mixture was sterilized by filtering through a 0.22 μm microporous membrane, and the filtrate Put it in the tray of the freeze dryer, cool slowly to -32°C, and maintain the initial freezing at -32°C for 2.5 hours, then quickly cool to -50°C, vacuumize, and rai...
Embodiment 3
[0061] prescription:
[0062] Fleroxacin 206.08g
[0063] Mannitol 123g
[0064] Lactic acid 68.35ml
[0065] Add water for injection to 2000ml
[0066]
[0067] Total 1000 bottles
[0068] Preparation process: Add 206.08g of fleroxacin and 123g of mannitol into 1600mL of water for injection, stir, then dropwise add 68.35mL of lactic acid to the above mixture, stir until fleroxacin is completely dissolved, then add 0.53g of Activated carbon, stirred at room temperature for 30 minutes, filtered through a 0.45 μm microporous membrane to remove the activated carbon, then added water for injection to 2000 mL, stirred thoroughly for 20 minutes to mix the solution evenly, and the obtained mixture was sterilized by filtering through a 0.22 μm microporous membrane, and the filtrate was placed in In the lyophilizer tray, cool slowly to -35°C, and maintain -35°C for 2.5 hours, then quickly cool to -45°C, vacuum, and heat up to -5°C within 12 hours, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com