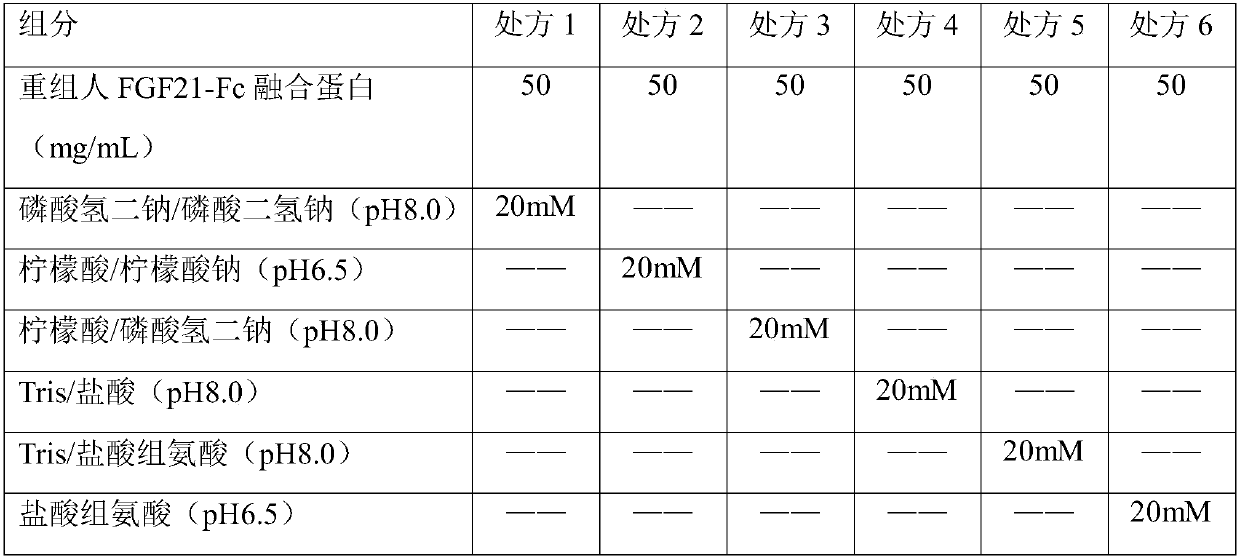
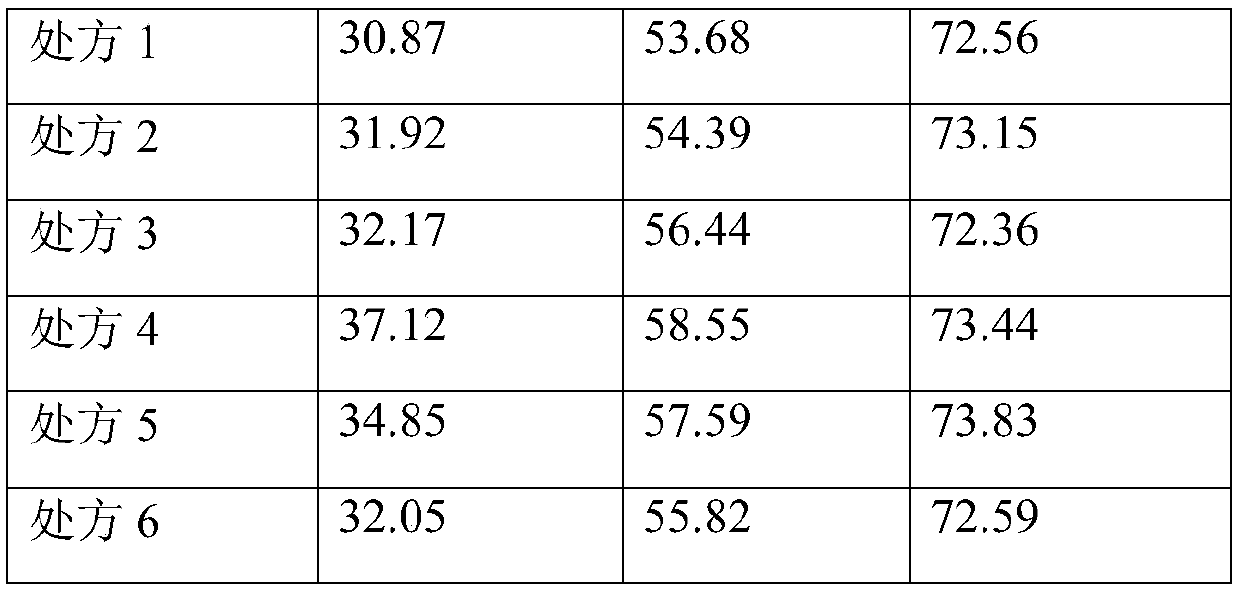
Freeze-dried powder preparation of metabolic regulation fusion protein
A fusion protein and metabolic regulation technology, which can be used in metabolic diseases, freeze-dried delivery, peptide/protein components, etc., and can solve problems such as easy aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] formula:
[0087] FGF21-Fc fusion protein 50mg / ml sucrose 250mM Polysorbate 80 0.02% (W / V) Tris / histidine hydrochloride 20mM pH 8.0
[0088] Preparation process: Weigh the prescription amount of Tris and dissolve it with an appropriate amount of water for injection, adjust the pH to 8.0 with histidine hydrochloride, take a sample to test the pH and endotoxin, and then accurately measure the prescription amount of polysorbate 80, sucrose and recombinant human Add the original solution of FGF21-Fc fusion protein to Tris / histidine hydrochloride buffer solution, mix evenly to obtain the semi-finished solution; filter the semi-finished solution with a 0.22μm filter membrane, and fill it after passing the endotoxin test, as described in the table below Lyophilize with the lyophilization program of Table 9.
[0089] Table 9 Example 1 freeze-drying program
[0090]
[0091]
Embodiment 2
[0093] formula:
[0094] FGF21-Fc fusion protein 30mg / ml Trehalose 50mM Polysorbate 80 0.04% (W / V) Tris / hydrochloric acid 10mM pH 8.5
[0095] Preparation process: Weigh the prescription amount of Tris, dissolve it with an appropriate amount of water for injection, adjust the pH to 8.5 with hydrochloric acid, take a sample to test the pH and endotoxin, and then accurately measure the prescription amount of polysorbate 80, trehalose and recombinant human FGF21- The Fc fusion protein stock solution was added to Tris-hydrochloric acid buffer solution, mixed evenly to obtain a semi-finished product solution; the semi-finished product solution was sterile filtered with a 0.22 μm filter membrane, and the endotoxin was tested to be qualified, then filled, and lyophilized according to the freeze-drying procedure in Table 10.
[0096] Table 10 Example 2 freeze-drying program
[0097]
Embodiment 3
[0099] formula:
[0100]
[0101]
[0102] Preparation process: Weigh the prescription amount of Tris and dissolve it with an appropriate amount of water for injection, adjust the pH to 7.5 with histidine hydrochloride, take a sample to test the pH and endotoxin, and then accurately measure the prescription amount of polysorbate 80, mannitol and recombinant human Add the FGF21-Fc fusion protein stock solution into Tris-hydrochloric acid buffer solution, mix evenly to obtain a semi-finished product solution; filter the semi-finished product solution with a 0.22 μm filter membrane, fill it after passing the endotoxin test, and follow the freeze-drying procedure described in Table 11 lyophilized.
[0103] Table 11 Example 3 freeze-drying program
[0104]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com