Alkyl phospholipid derivatives with reduced cytotoxicity and uses thereof
A technology of alkyl phospholipids and derivatives, applied in medical preparations containing active ingredients, organic active ingredients, antiviral agents, etc., can solve problems such as long courses of treatment and serious side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
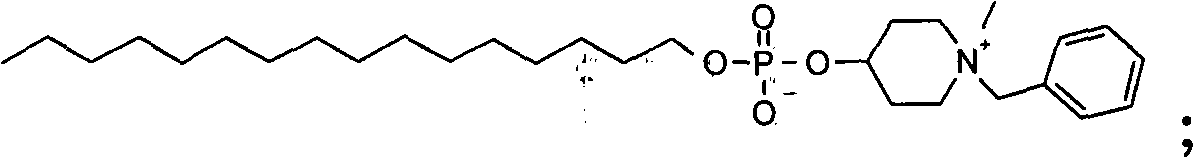
[0865] Example 1: Compound 1
[0866] 1-Benzyl-1-methyl-piperidin-4-yl phosphate hexadecyl ester
[0867] After methylation of 1-benzyl-piperidin-4-ol according to general method 2, 3.1 g were obtained starting from cetyl-1-ol and 1-benzyl-piperidin-4-ol according to general method 4 ( 6%) compound 1.
[0868]1 H-NMR (600MHz, CDCl 3 -d1, 300K): δ=7.59 (2H, d), 7.47-7.40 (3H, m), 4.78 (2H, broad peak-s), 4.55 (1H, broad peak-s), 3.86-3.77 (4H, m), 3.56 (2H, d), 3.13 (3H, s), 2.27 (2H, d), 2.13 (2H, t), 1.57 (2H, quintet), 1.31-1.18 (26H, m), 0.88 (3H,t)ppm
[0869] ESI-MS: Found: m / z 510.4 [M+H], Calculated: 510.7 g / mol
Embodiment 2
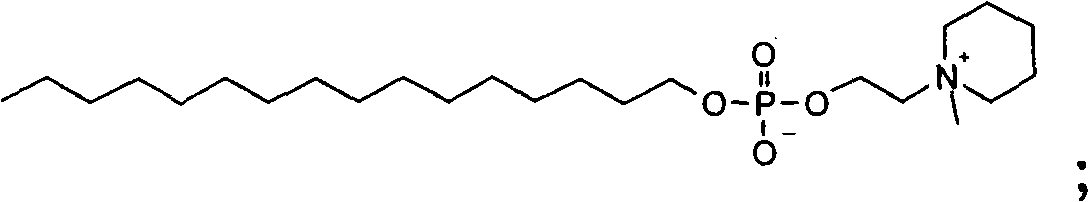
[0870] Example 2: Compound 2
[0871] Cetyl phosphate 2-(1-methyl-piperidin-1-yl)-ethyl ester
[0872] After methylation of 2-piperidin-1-yl-ethanol according to general method 2, 5.8 g (13% ) Compound 2.
[0873] 1 H-NMR (600MHz, CDCl 3 -d1, 300K): δ=4.33 (2H, broad peak-s), 3.89-3.81 (4H, m), 3.70 (2H, m), 3.57-3.53 (2H, m), 3.37 (3H, s), 1.93-1.86 (4H, m), 1.74-1.68 (2H, m), 1.59 (2H, quintet), 1.35-1.19 (26H, m), 0.88 (3H, s) ppm
[0874] ESI-MS: Found: m / z 448.3 [M+H], Calculated: 448.6 g / mol
Embodiment 3
[0875] Example 3: Compound 3
[0876] 2-(1-Aza-bicyclo[2.2.2]oct-1-yl)-ethyl hexadecyl phosphate
[0877] After alkylation of chinuclidine according to general method 1, 2,0 g (5%) of compound 3 were obtained according to general method 4 starting from cetyl-1-ol and 1-(2-hydroxy-ethyl)-chinuclidine.
[0878] 1 H-NMR (600MHz, CDCl 3 -d1, 300K): δ=4.29 (2H, broad peak-s), 3.84 (2H, q), 3.73 (8H, m), 2.17 (1H, m), 1.60 (2H, quintet), 1.34- 1.22(26H, m), 0.88(3H, t)ppm
[0879] ESI-MS: Found: m / z 460.5 [M+H], Calculated: 460.7 g / mol
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com