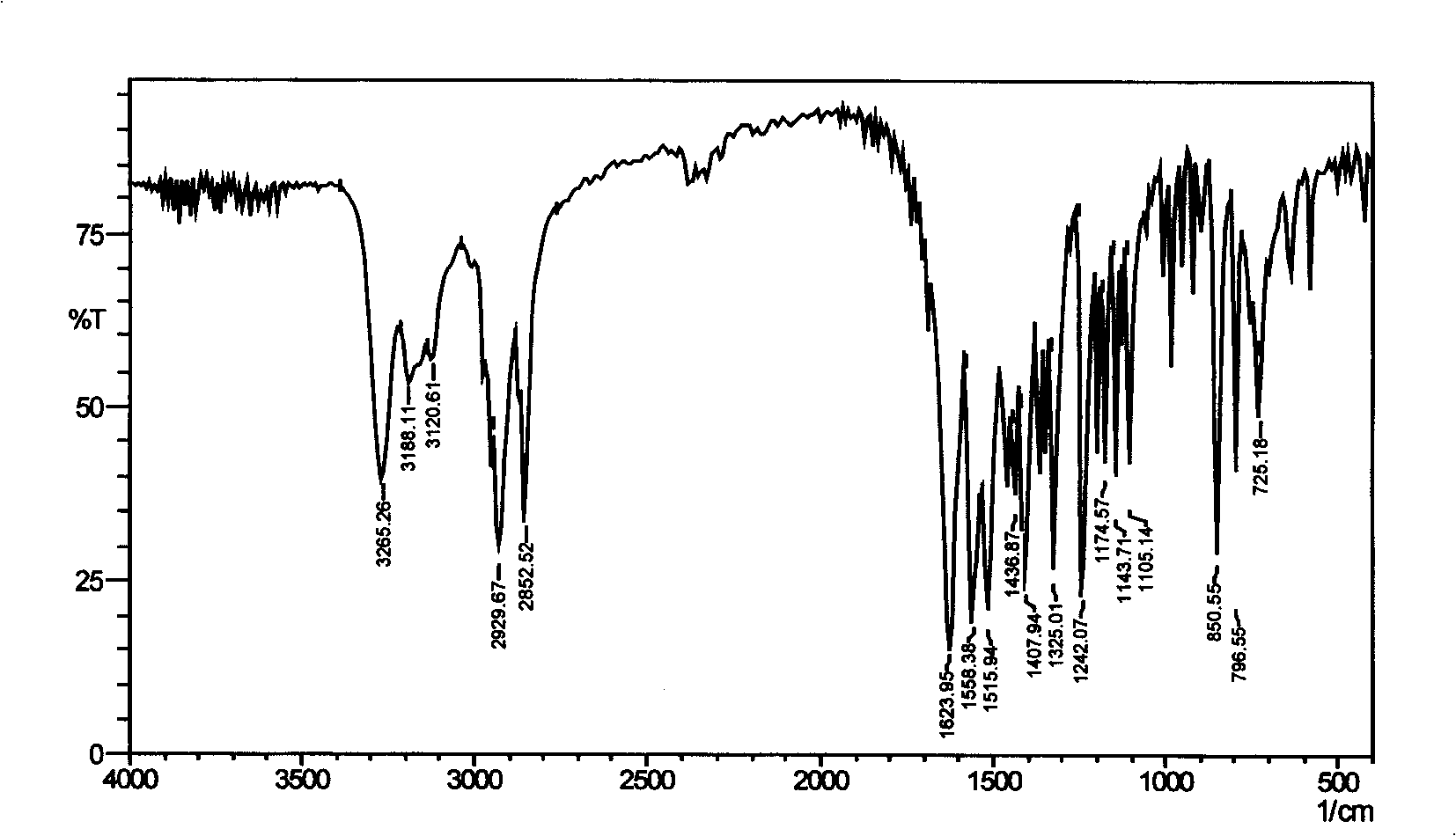
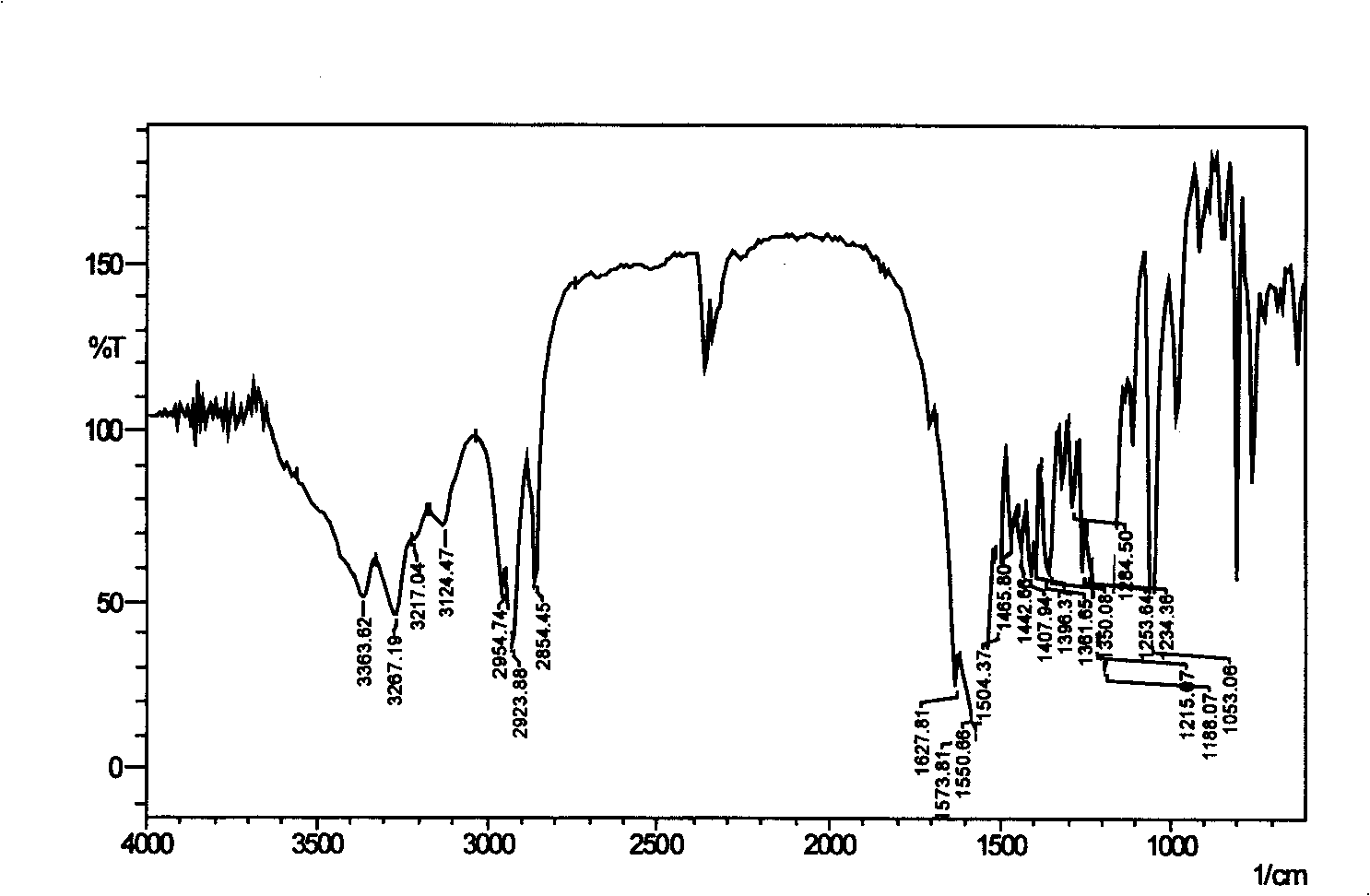
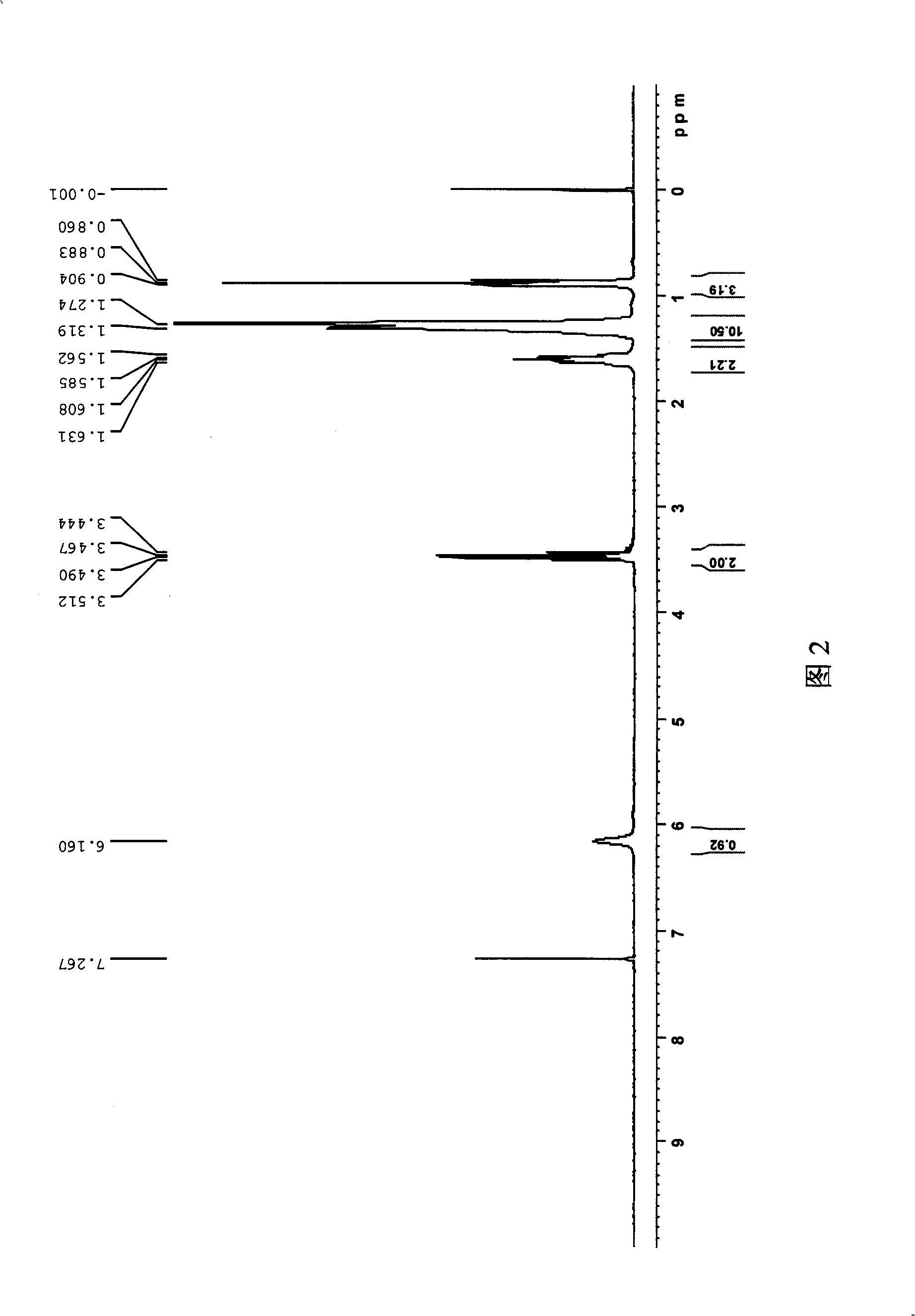
Gemini surfactant containing triazine ring
A surfactant and triazine ring technology, applied in the field of anionic Gemini surfactants, can solve the problems of unsatisfactory surface activity and other properties, long synthesis steps, expensive raw materials, etc., and achieve excellent surface activity and high The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The synthesis of surfactant (I) represented by the following formula:
[0053]
[0054] 1. Synthesis of 2,4-dichloro-6-n-octylamino-1,3,5-s-triazine
[0055] Add 100mL of toluene to a 250mL four-necked bottle, keep the temperature between 0 and 5°C with an ice bath, add 18.45g of cyanuric chloride, stir to dissolve it, and slowly drop 50mL of toluene solution in which 15.48g of n-octylamine is dissolved , maintain a pH value of 7-8 during the reaction.
[0056] Adopt thin-layer chromatography (TLC) method to track the reaction process, with toluene: acetone=1: 1 (V / V) as developing solvent, the reaction solution is carried out thin-layer chromatography, when the spot of cyanuric chloride disappears substantially , that is to say, the reaction has reached the end point, and the reaction ends.
[0057] After the reaction finished, the reaction solution was moved to a separatory funnel to stand for layering, and the separated organic layer was washed with 0.1mol / L hyd...
Embodiment 2
[0069] Synthesis of surfactant (II) represented by the following formula:
[0070]
[0071] 1. Synthesis of 2,4-dichloro-6-n-octylamino-1,3,5-s-triazine
[0072] With embodiment 1.
[0073] 2. Synthesis of 2-chloro-4-sulfoethylamino-6-n-octylamino-1,3,5-s-triazine
[0074] With embodiment 1.
[0075] 3. Synthesis of Gemini Surfactant (II)
[0076] Add 1.11g of propylenediamine and 50mL of water into the reactor, raise the temperature to 80-110°C, slowly add 9.69g of 2-chloro-4-sulfoethylamino-6-n-octylamino-1,3 into the reactor dropwise, 50 mL of aqueous solution of 5-s-triazine, and react at constant temperature for a certain period of time.
[0077] Carry out thin-layer chromatography with methanol:toluene=6:1 (V / V) as developing solvent, when 2-chloro-4-sulfoethylamino-6-n-octylamino-1,3,5-s-triazine When the spot disappears substantially, can think that reaction finishes, obtains Gemini tensio-active agent (II).
Embodiment 3
[0079] The synthesis of surfactant (III) represented by the following formula:
[0080]
[0081] 1. Synthesis of 2,4-dichloro-6-n-octylamino-1,3,5-s-triazine
[0082] With embodiment 1.
[0083] 2. Synthesis of 2-chloro-4-sulfoethylamino-6-n-octylamino-1,3,5-s-triazine
[0084] With embodiment 1.
[0085] 3. Synthesis of Gemini Surfactant (III)
[0086] Add 1.74g of hexamethylenediamine and 50mL of water into the reactor, raise the temperature to 80-110°C, slowly add 9.69g of 2-chloro-4-sulfoethylamino-6-n-octylamino-1,3 into the reactor dropwise, 50 mL of aqueous solution of 5-s-triazine, and react at constant temperature for a certain period of time.
[0087] Carry out thin-layer chromatography with methanol:toluene=6:1 (V / V) as developing solvent, when 2-chloro-4-sulfoethylamino-6-n-octylamino-1,3,5-s-triazine When the spot disappears substantially, can think that reaction finishes, obtains Gemini tensio-active agent (III).
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com