Quality control method of ginseng and astragalus injection for strengthening body
A technology of Shenqi Fuzheng and injection, which is applied in the field of fingerprint control of Shenqi Fuzheng injection, and can solve the problems of general practical limitations of the method, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1, the establishment of the standard fingerprint of Shenqi Fuzheng Injection (A method)
[0041] 1. Preparation of standard test solution
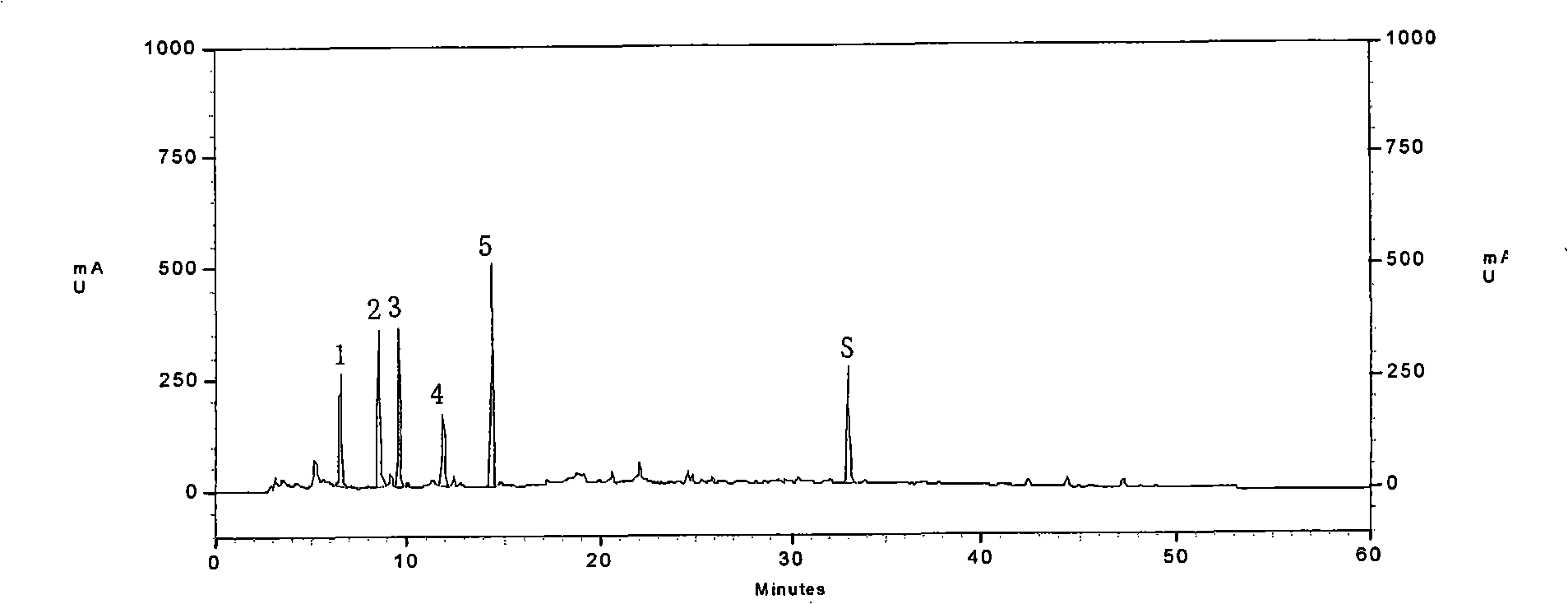
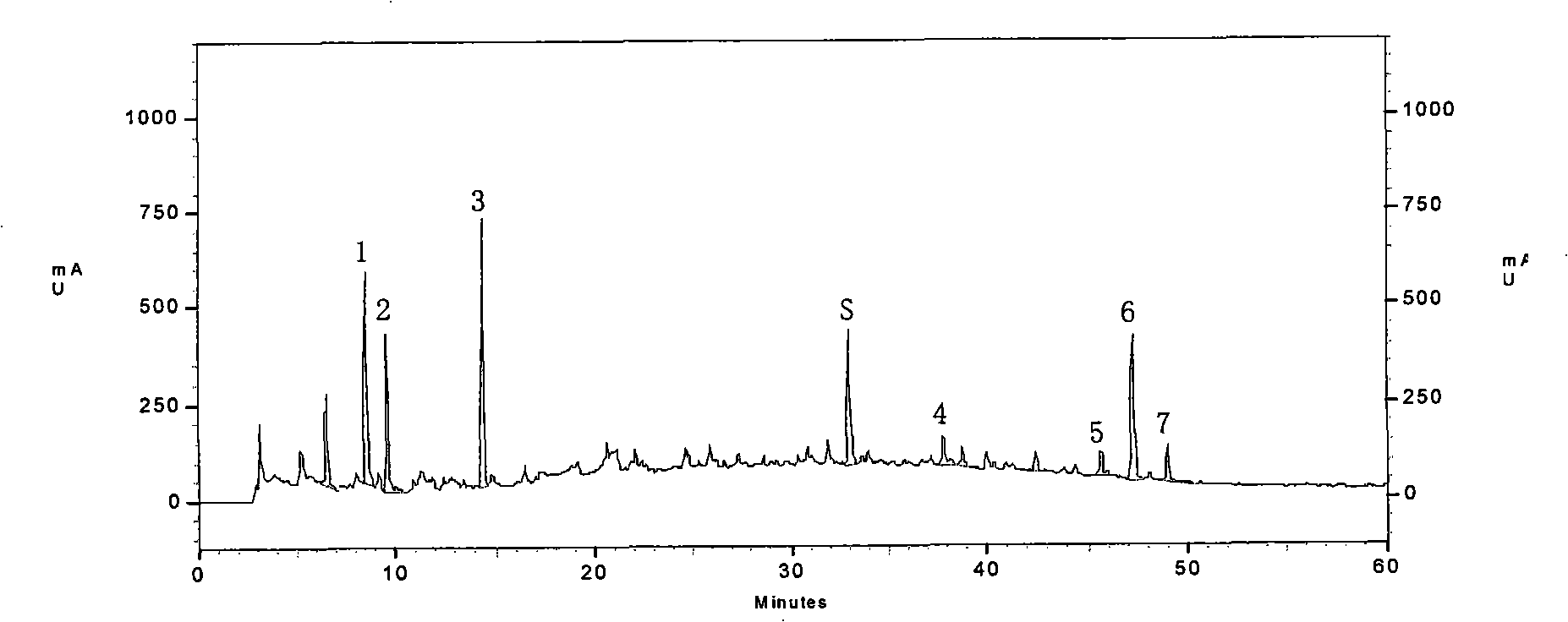
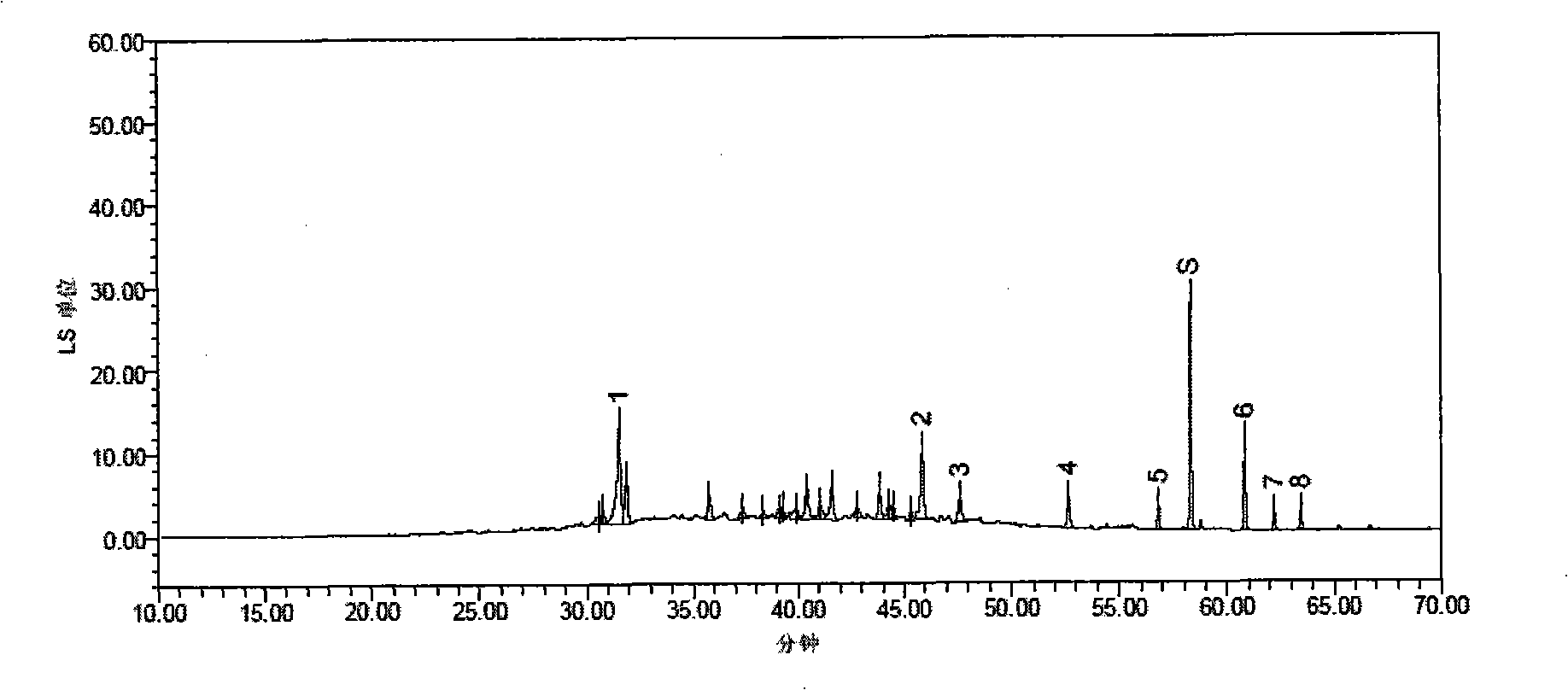
[0042] ①Fingerprint of phenolic acids: Precisely measure 25ml of Shenqi Fuzheng injection sample, put it in a water bath to concentrate to about 5ml, and slowly pass it through the AB-8 macroporous adsorption resin column (the inner diameter of the column is 1.5cm, and the column length is 20cm). Elute with 70ml of water, discard the water eluate, continue to elute with 80ml of 70% ethanol, collect the 70% ethanol eluate, put it in a water bath and concentrate to dryness, add water to dissolve the residue and transfer it to a 10ml measuring bottle, add water to dilute to Scale, shake well, filter with 0.45μm microporous membrane, that is.
[0043] ②Fingerprint of saponins: Precisely measure 200ml of Shenqi Fuzheng injection sample, put it in a water bath and concentrate it to about 10ml, slowly pass it through the AB-8 m...
Embodiment 2
[0095] Embodiment 2. The establishment of the standard fingerprint of Shenqi Fuzheng Injection (B method)
[0096] 1. Preparation of standard test solution
[0097] (1) Test sample of phenolic acid fingerprints: Accurately measure 25ml of Shenqi Fuzheng injection sample, extract four times with water-saturated n-butanol, 20ml each time, combine n-butanol solution, let stand, and wait for complete clarification , discard the remaining water layer, put the n-butanol solution in a water bath and concentrate to dryness, add water to dissolve the residue, transfer it to a 10ml measuring bottle, add water to dilute to the mark, shake well, and use a microporous membrane filter (water phase, 0.45 μm ) filter, that is.
[0098](2) Test samples of saponins fingerprints: Accurately measure 200ml of Shenqi Fuzheng injection sample, put in a water bath and concentrate to about 20ml, extract with n-butanol saturated with water four times, 25ml each time, combine the n-butanol solution, W...
Embodiment 3
[0146] Embodiment 3, this method is applied to the quality control of Shenqi Fuzheng Injection
[0147] Select commercially available Astragalus Injection, Salvia Infusion, and Shenqi Fuzheng Injection, measure its fingerprints according to the A method of Example 1, and compare them with standard fingerprints. If the similarity is greater than 0.9, it is Shenqi Fuzheng Injection. The rest are all lower than 0.9, which can be used to distinguish the authenticity of Shenqi Fuzheng Injection.
[0148] Astragalus Injection:
[0149] The fingerprints of phenolic acids and saponins at 208nm and 266nm are as follows: Figure 7A one Figure 7C , and their similarities are 0.409, 0.315, and 0.611, respectively.
[0150] Salvia Infusion:
[0151] The fingerprints of phenolic acids and saponins at 208nm and 266nm are as follows: Figure 8A one Figure 8C , and their similarities are 0.292, 0.257, and 0.000, respectively.
[0152] Shenqi Fuzheng Injection:
[0153] The fingerprin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com