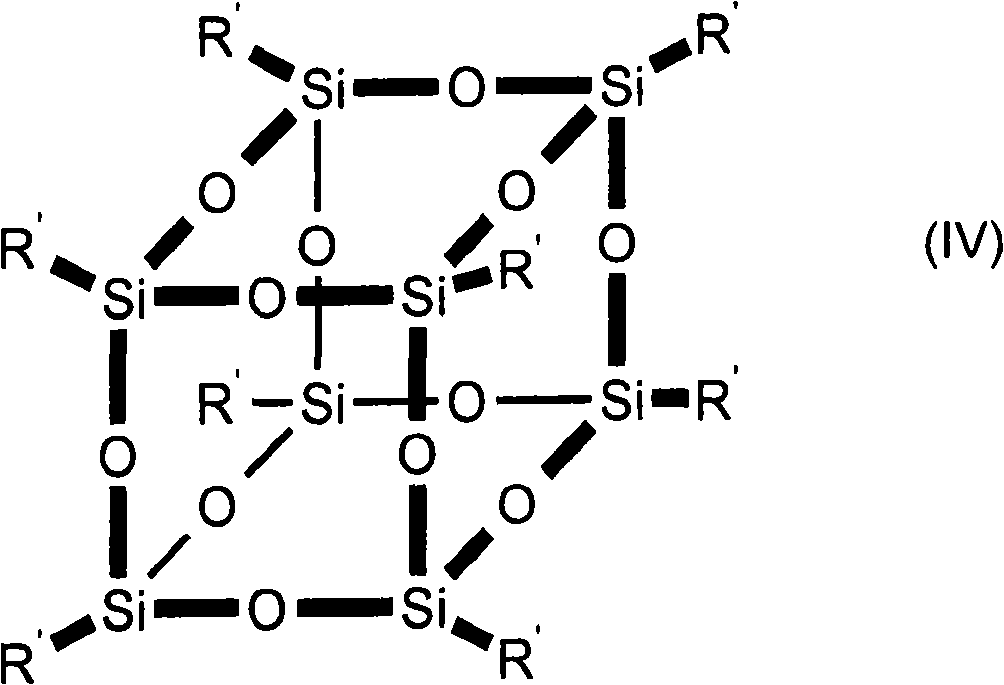
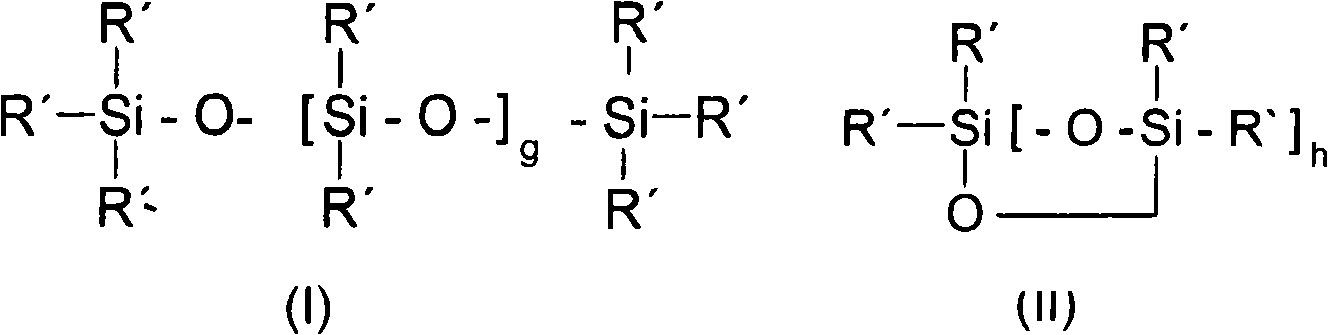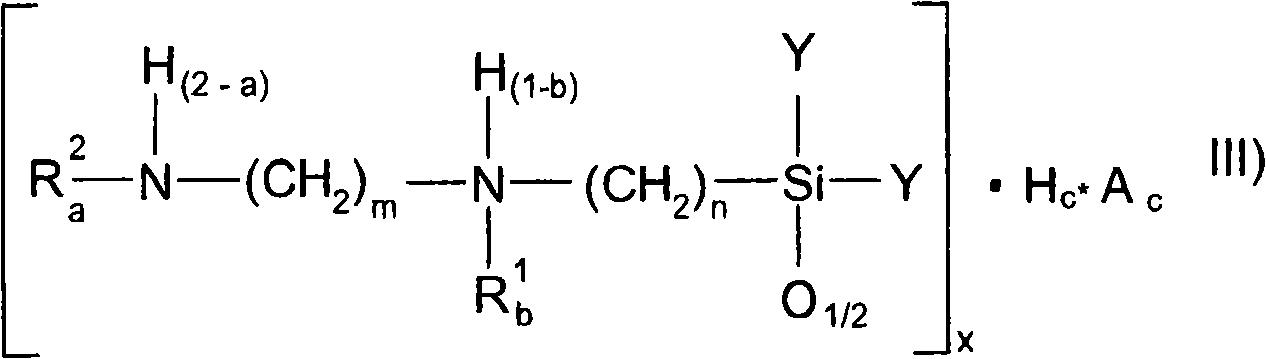Amido functional group polysiloxane and composition based on the same
A polysiloxane and group technology, applied in the field of stable compositions with low chloride content, can solve problems such as substances being difficult to dissolve in water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] Condensed by hydrolysis [CH 2 =CH-C 6 h 4 -CH 2 -NH-(CH 2 ) 2 NH(CH 2 ) 3 Si(OCH 3 ) 3 ]HCl(VIb) to prepare amino-functional polysiloxane
[0132] In a heatable 2-1-three-necked flask equipped with a nitrogen stacking device, a distillation device and a dropping funnel, 750 g of a 50% by weight methanol solution of VI (3.74% by weight of silicon) was preliminarily placed. Next, 27 g of water (corresponding to 1.5 mol of water per mol of silicon used) was slowly added with stirring. 27 g of methanol were then removed from the reaction mixture by distillation at ambient pressure. The solution was then heated to 60°C for about 3 hours. The solution had a silicon content of 3.74% by weight.
Embodiment 2
[0134] Condensation by hydrolysis [C 6 h 5 -CH 2 -NH-(CH 2 ) 2 NH(CH 2 ) 3 Si(OCH 3 ) 3 ]HCl(VIc) to prepare functionalized aminopolysiloxane
[0135] Into a heatable 2-1-three-necked flask equipped with a nitrogen stacking device, a distillation device and a dropping funnel, 697 g of a 50% by weight methanol solution of VII (4.01% by weight of silicon) was placed in advance. Next, 27 g of water (corresponding to 1.5 mol of water per mol of silicon used) was slowly added with stirring. 27 g of methanol were then removed from the reaction mixture by distillation at ambient pressure. The solution was then heated to 60°C for about 3 hours. The solution had a silicon content of 4.01% by weight.
Embodiment 3
[0137] NH condensed by hydrolysis 2 -(CH 2 ) 2 NH(CH 2 ) 3 Si(OCH 3 ) 3 (VId) and with CH 2 =CH-C 6 h 4 -CH 2 Preparation of Functionalized Aminopolysiloxane by Reaction of -Cl(VIIa)
[0138] 222 g of aminoethylaminopropyltrimethoxysilane was pre-placed in a heatable 1-1-three-necked flask equipped with a nitrogen stacking device, a reflux cooler and a dropping funnel. Then 27 g of water (corresponding to 1.5 mol per mol of silicon used) were added slowly with stirring. The reaction mixture was heated to about 60°C. The temperature was maintained by heating for 1 hour. Then 105 g of methanol was added. Then 152.5 g of vinylbenzyl chloride mixed with 104.5 g of methanol was slowly added with stirring. The temperature was maintained at 64°C by cooling (during the addition) and subsequent heating for about 3 hours. After cooling, the silicon content was diluted to 3.74% by weight with 138 g of methanol.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Ignite | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com