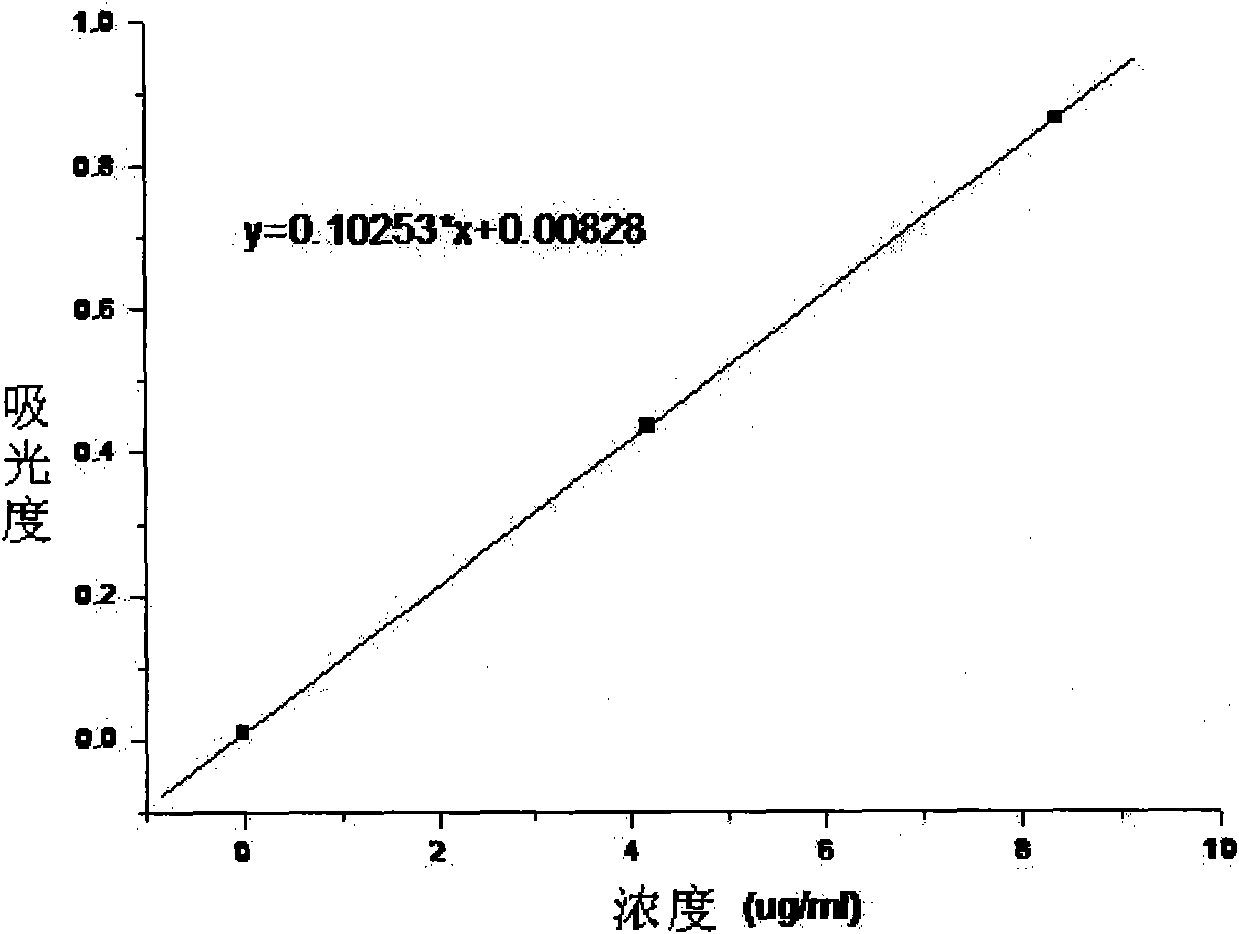Electroactive polymer with water solubility and biological degradability, preparation method thereof and application thereof
An electroactive polymer and biodegradable technology, applied in the field of medical devices, can solve the problems of easy induction of chronic inflammation, failure to meet the requirements of water solubility, poor solubility, etc., and achieve the effect of simple process and promotion of tissue regeneration and repair
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1. Sample
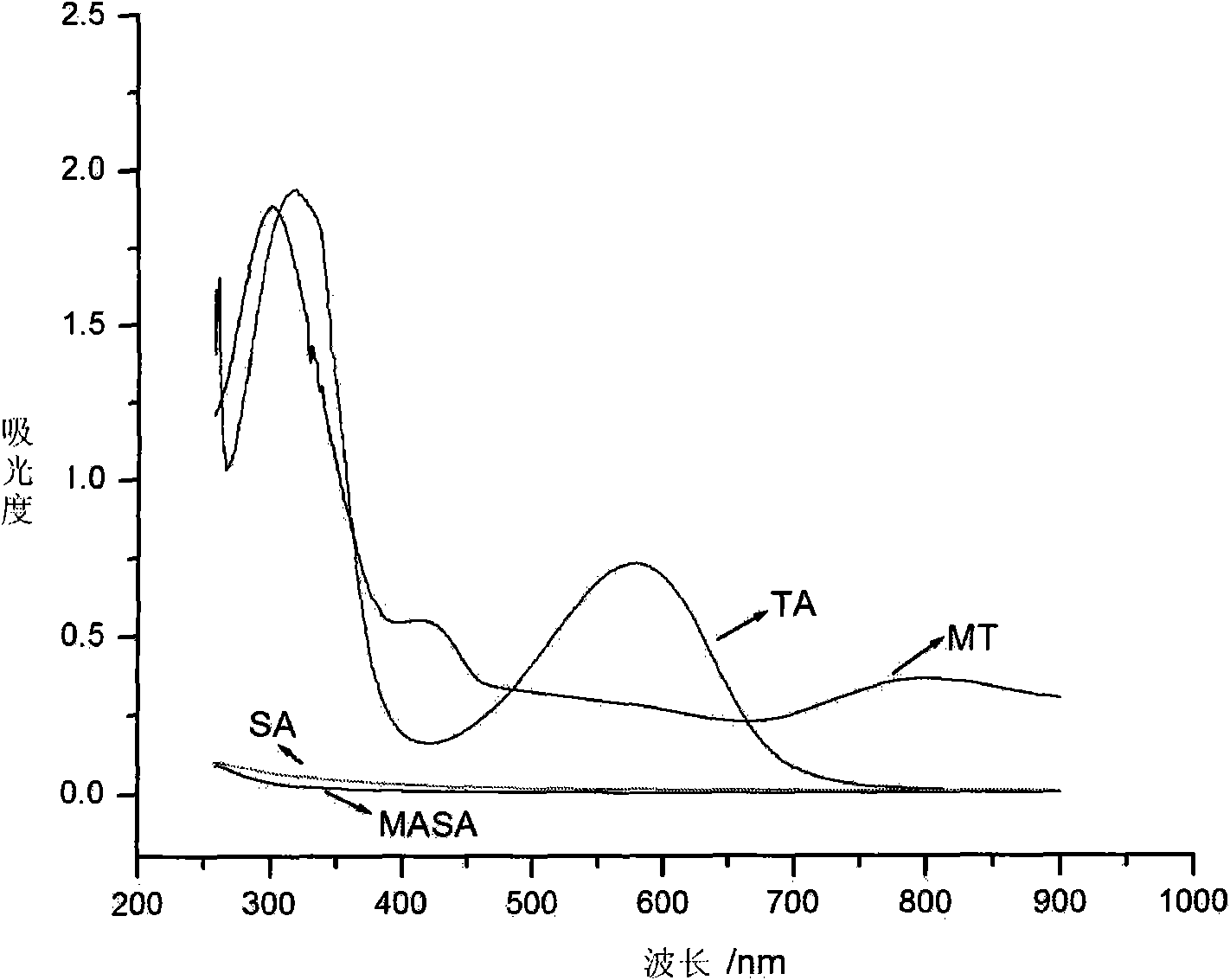
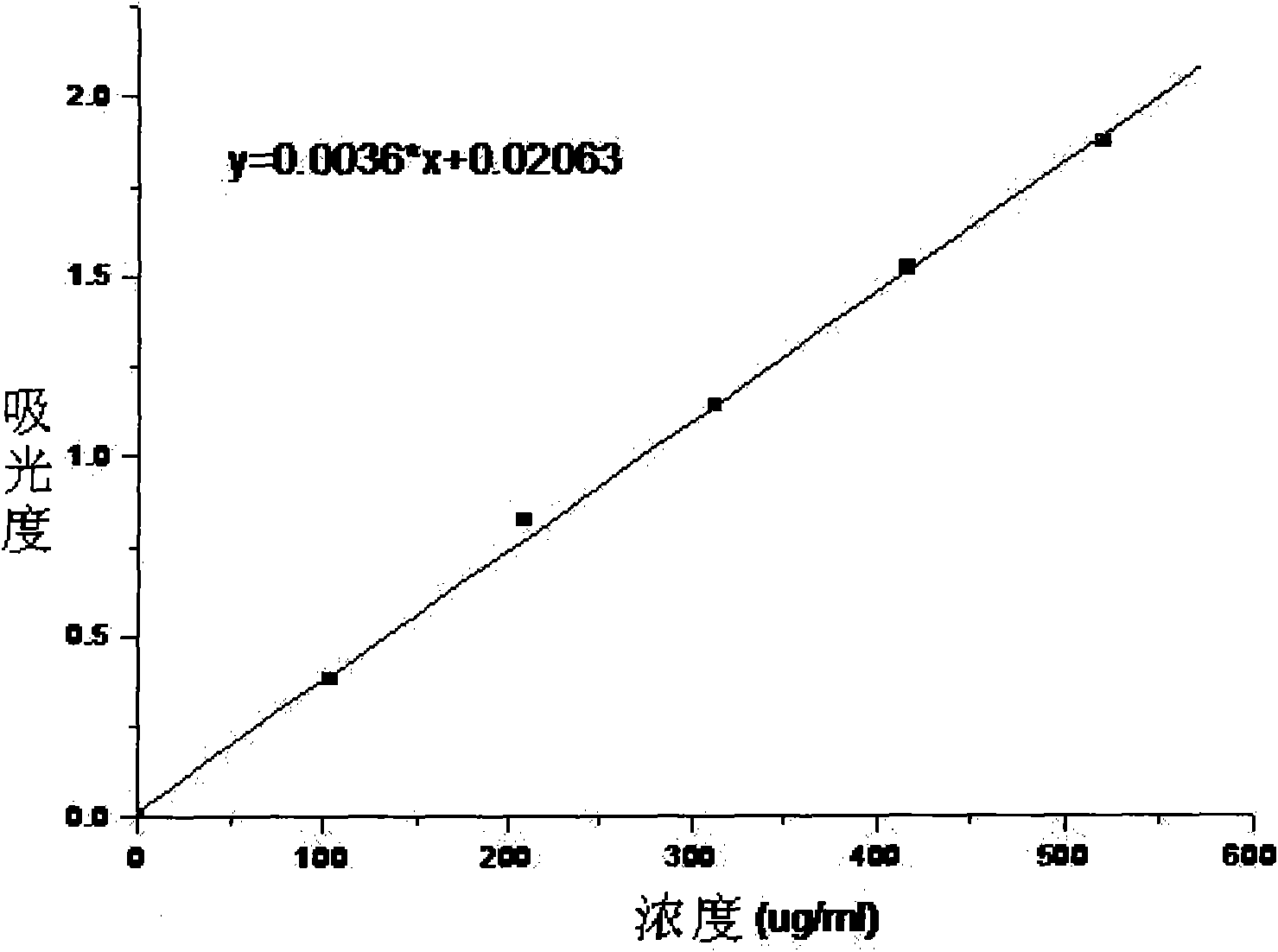
[0040] The water-soluble and biodegradable electroactive polymer provided in this example is prepared by the following method: sodium periodate is used to oxidize sodium alginate to prepare polyaldehyde-based sodium alginate MASA, and the method of oxidative coupling is adopted Prepare the intrinsic state benzene / amine-terminated aniline tetramer, and obtain the fully reduced benzene / amine-terminated aniline tetramer TA-NH after reducing the intrinsic state benzene / amine-terminated aniline tetramer 2 , through the all-reduced benzene / amine capping aniline tetramer TA-NH 2 The terminal amino groups on the polyaldehyde sodium alginate MASA react with the aldehyde groups on the polyaldehyde sodium alginate MASA, and the fully reduced benzene / amine-terminated aniline tetramer TA is introduced into the polyaldehyde sodium alginate MASA to obtain aniline oligomer-modified polyaldehyde Base sodium alginate TA-MASA, its structural formula is as follows:
[0041] A...
Embodiment 2
[0078] The water-soluble and biodegradable electroactive polymer provided in this example is prepared by the following method: sodium periodate is used to oxidize sodium alginate to prepare polyaldehyde-based sodium alginate MASA, and the method of oxidative coupling is adopted Prepare the intrinsic state benzene / amine-terminated aniline oligomer, and obtain the fully reduced benzene / amine-terminated aniline tetramer TA-NH after reducing the intrinsic state benzene / amine-terminated aniline oligomer 2 , the fully reduced benzene / amine-terminated aniline octamer OcA-NH was prepared by adding aniline tetramer to phenylhydrazine containing ferric chloride 2 , capping the aniline octamer OcA-NH via the all-reduced benzene / amine 2The terminal amino group on the polyaldehyde group reacts with the aldehyde group on the polyaldehyde sodium alginate MASA, and the fully reduced benzene / amine-terminated aniline octamer OcA is introduced into the polyaldehyde sodium alginate MASA to obtain...
Embodiment 3
[0092] The water-soluble and biodegradable electroactive polymer provided in this example is prepared by the following method: sodium periodate is used to oxidize sodium alginate to prepare polyaldehyde-based sodium alginate MASA, and the method of oxidative coupling is adopted Prepare the intrinsic state benzene / amine-terminated aniline oligomer, and obtain the fully reduced benzene / amine-terminated aniline oligomer OA-NH after reducing the intrinsic state benzene / amine-terminated aniline oligomer 2 , capping the aniline oligomer OA-NH via the fully reduced benzene / amine 2 The terminal amino group on the polyaldehyde group reacts with the aldehyde group on the polyaldehyde sodium alginate MASA, and the fully reduced benzene / amine-terminated aniline oligomer OA is introduced into the polyaldehyde sodium alginate MASA to obtain the polyaldehyde modified by the aniline oligomer Base sodium alginate OA-MASA, its structural formula is as follows:
[0093] Among them, the structur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com