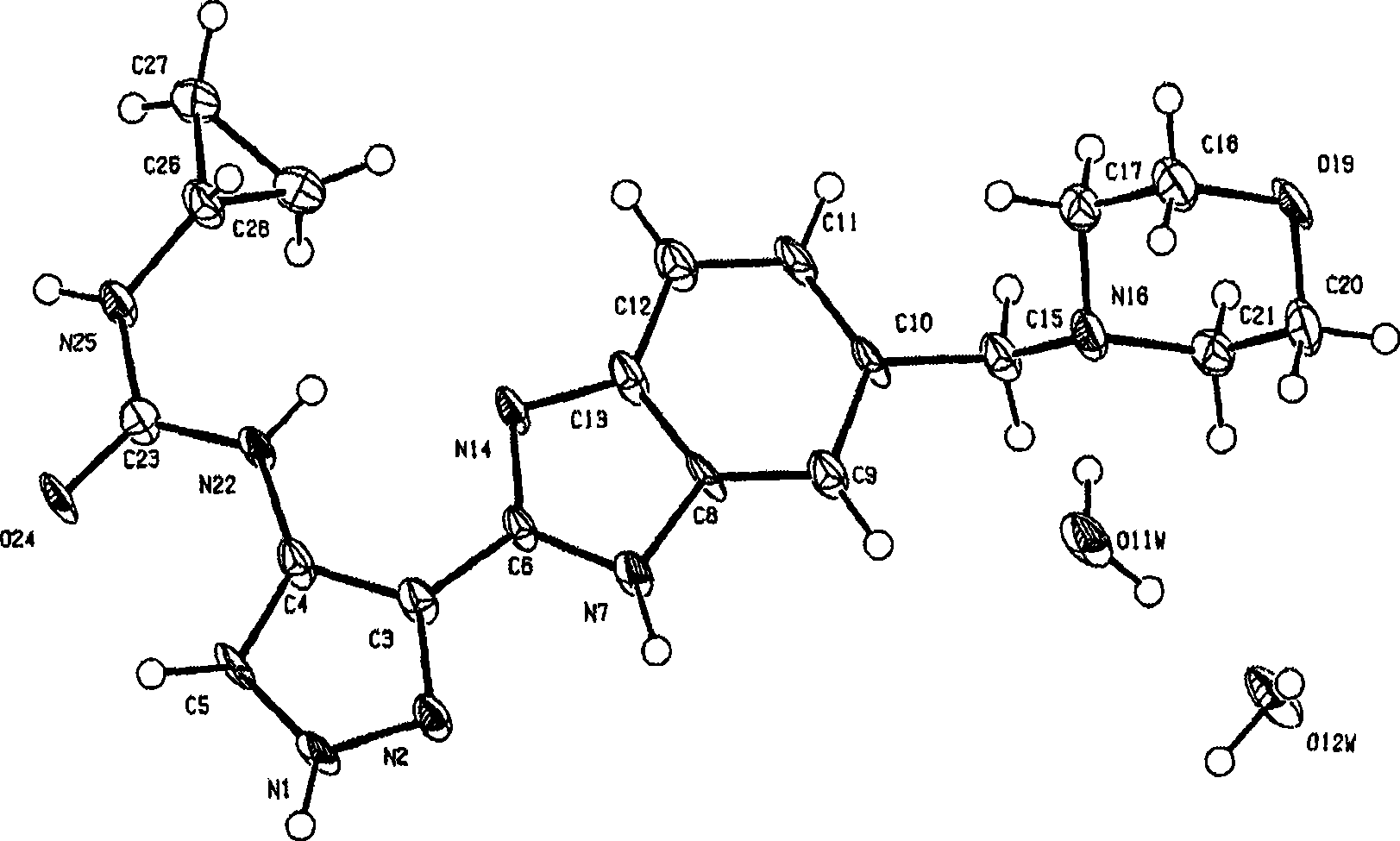
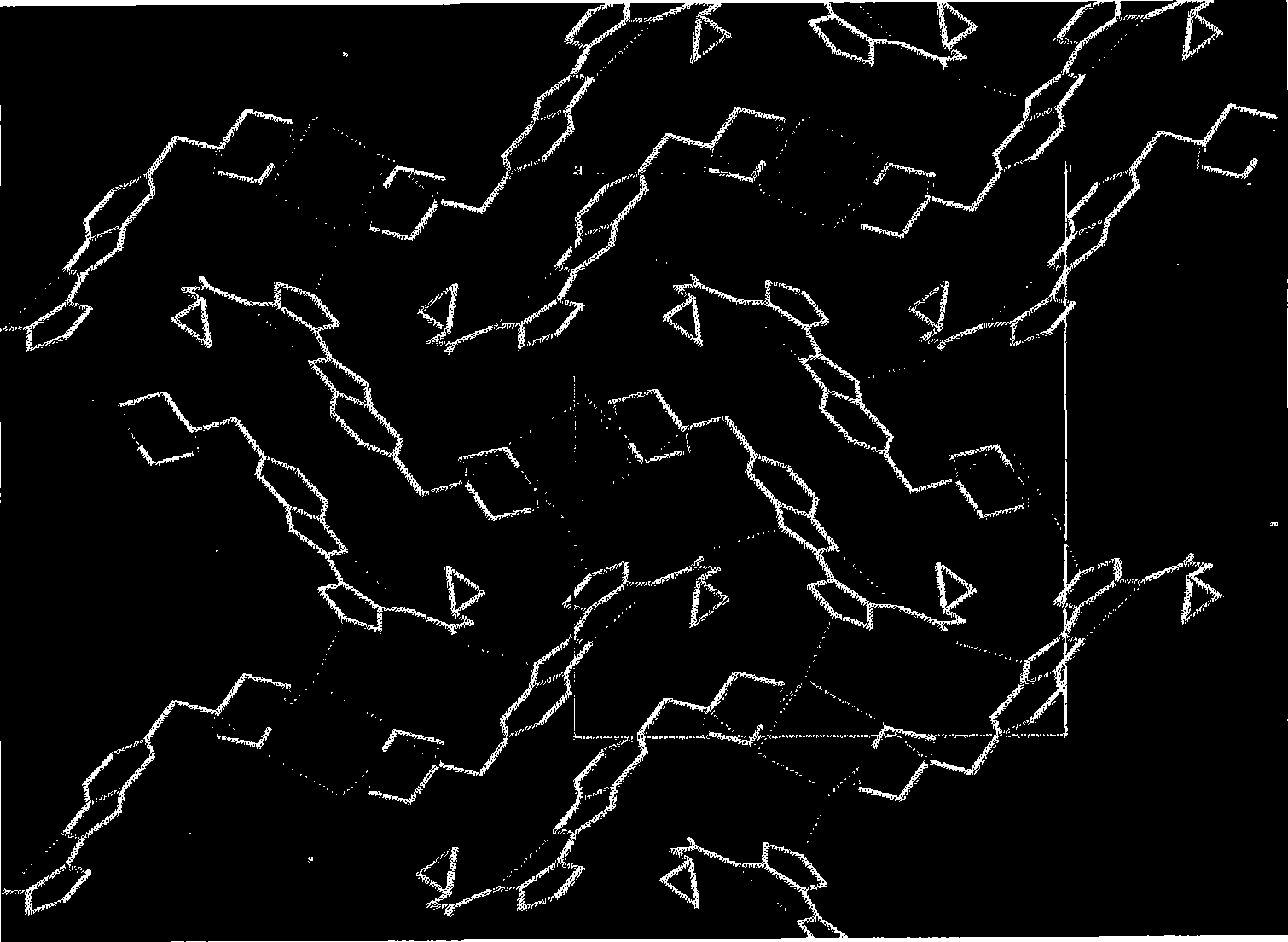
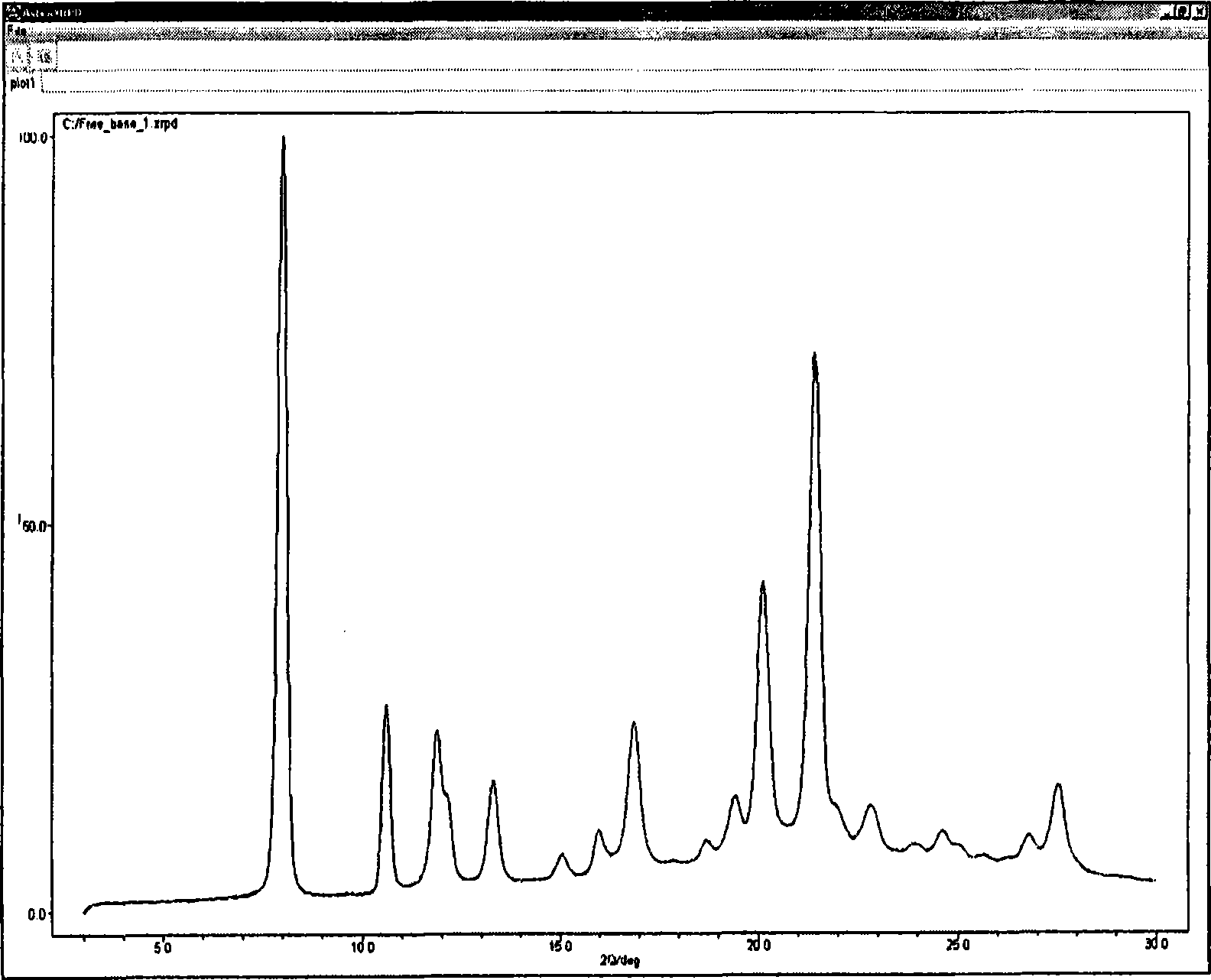
Pyrazole compounds that modulate the activity of CDK, GSK and aurora kinases
A technology of compounds and oxides, applied in disease states or diseases, in the field of new compounds with kinase inhibitory or regulatory activities, which can solve the problems of not disclosing the activity of CDK kinases or GSK kinases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1016] 5-cyano-2-methoxy-N-[3-(5-morpholin-4-ylmethyl-1H-benzimidazole -2-yl)-1H-pyrazol-4-yl)-benzamide
[1017] 1A. Synthesis of (3,4-Dinitro-phenyl)-morpholin-4-yl-methanone (methanone)
[1018]
[1019] A mixture of 3,4-dinitrobenzoic acid (10.0 g) and thionyl chloride (30 ml) was heated under reflux for 2 hours, cooled to ambient temperature and excess thionyl chloride was removed by azeotroping with toluene. The residue was taken up in THF (100ml) and morpholine (4.1ml) and Et were simultaneously added to the mixture at 0°C 3 N (7.2ml). The mixture was stirred for 3 hours, water (100 ml) was added thereto, and then extracted with EtOAc. The organic part was washed with brine and dried (MgSO 4 ) And concentrate it under vacuum. The residue was recrystallized from MeOH to obtain (3,4-dinitro-phenyl)-morpholin-4-yl-methanone (8.23 g) as a yellow solid.
[1020] ( 1 H NMR(300MHz, DMSO-d 6 )δ 8.3(d, 1H), 8.3(s, 1H), ...
Embodiment 2
[1042] 6-Methyl-imidazo[2.1-b]thiazole-5-carboxylic acid [3-(5-morpholin-4-ylmethyl- Synthesis of 1H-benzimidazol-2-yl)-1H-pyrazol-4-yl)-amide
[1043]
[1044] The 6-methyl-imidazo[2.1-b]thiazole-5-carboxylic acid (Bionet) (61mg, 0.33mmol), 3-(5-morpholin-4-ylmethyl-1H-benzimidazole-2- A mixture of -1H-pyrazol-4-ylamine (100mg, 0.33mmol), EDC (77mg, 0.39mmol) and HOAt (54mg, 0.39mmol) was stirred in DMF (3ml) at 80°C for 1 hour, It was then stirred at ambient temperature for 20 hours. The mixture was reduced in vacuo and the residue was partitioned between EtOAc and saturated NaHCO. The organic part was washed with brine and dried (MgSO 4 ) And decrement it under vacuum. The residue was purified by preparative LC / MS to obtain 6-methyl-imidazo[2.1-b]thiazole-5-carboxylic acid [3-(5-morpholin-4-ylmethyl-1H-benzimidazole) -2-yl)-1H-pyrazol-4-yl]-amide (29 mg). (Alkaline LC / MS: R t 2.56[M+H] + 463).
Embodiment 3
[1046] 2-cyano-N-[3-(5-morpholin-4-ylmethyl-1H-benzimidazol-2-yl)- Synthesis of 1H-pyrazol-4-yl]-acetamide
[1047]
[1048] The cyano-acetic acid (23mg, 0.28mmol), 3-(5-morpholin-4-ylmethyl-1H-benzimidazol-2-yl)-1H-pyrazol-4-ylamine (70%, A mixture of 100 mg, 0.23 mmol), TBTU (89 mg, 0.28 mmol) and DMF (2 ml) was stirred at 25°C overnight. Then, the mixture was evaporated in vacuo. Purification by flash column chromatography, eluting with DCM-6% MeOH / DCM, gave 2-cyano-N-[3-(5-morpholin-4-ylmethyl-1H-) as a yellow solid. Benzimidazol-2-yl)-1H-pyrazol-4-yl]-acetamide (65 mg, 77%). (LC / MS (acid method / final compound): R t 4.61, [M+H] + 366).
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com