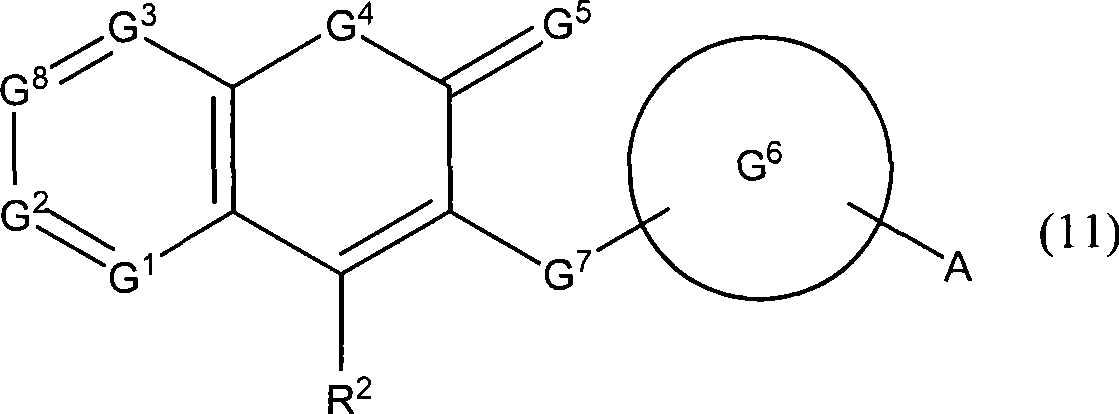
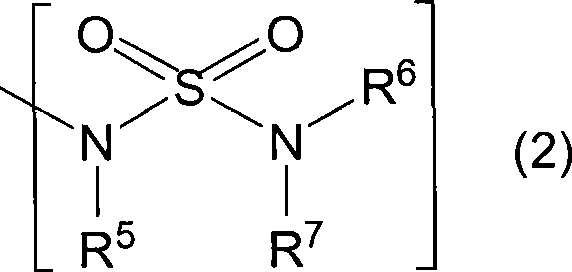
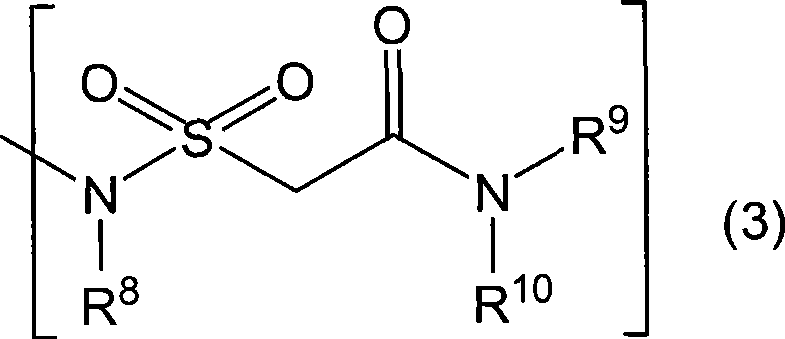
Novel coumarin derivative having antitumor activity
Technology of a compound, halogen atom, applied in the field of therapeutic agents for cell proliferative diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example
[0655] In the following production examples (synthesis examples), NMR analysis was performed using JNM-EX270 (270 MHz) manufactured by JEOL Corporation, JNM-GSX400 (400 MHz) manufactured by JEOL Corporation, or ARX-300 (300 MHz) manufactured by Bruka Corporation, and the NMR data were expressed in ppm (parts permillion, δ) are shown, referenced to the deuterium lock signal from the sample solvent.
[0656] In addition, mass spectrum data were obtained using JMS-DX303 manufactured by JEOL Corporation or JMS-SX / SX102A manufactured by JEOL Corporation, or a micro mass spectrometer equipped with Agilent 1100 gradient high performance liquid chromatography (Navigator manufactured by Finningan Corporation) manufactured by Agilent Technologies.
[0657] In the case of using a commercially available reagent, it was directly used for the reaction without performing pretreatments such as distillation and recrystallization. As a reaction solvent, when a commercially available solvent is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com