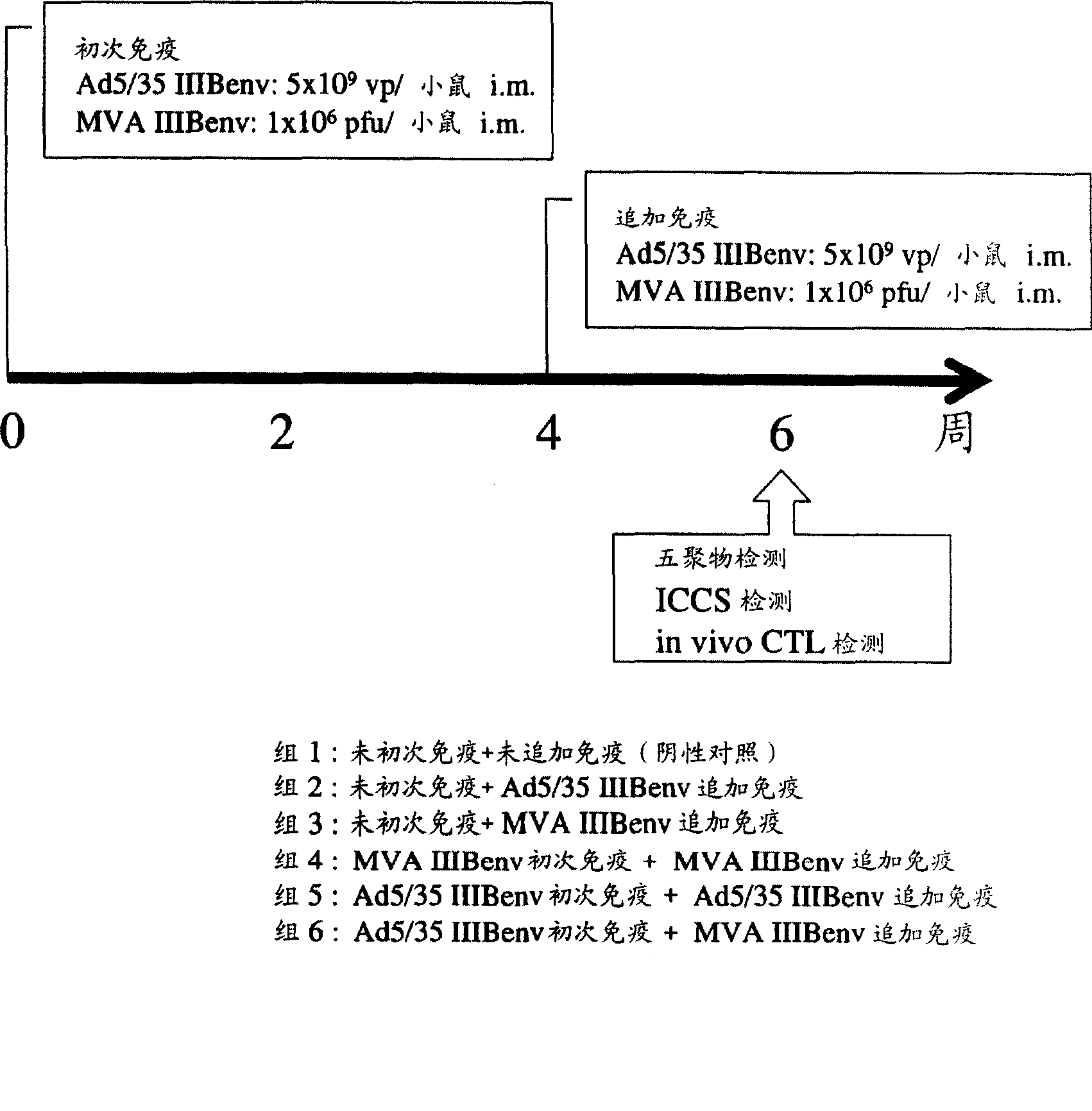
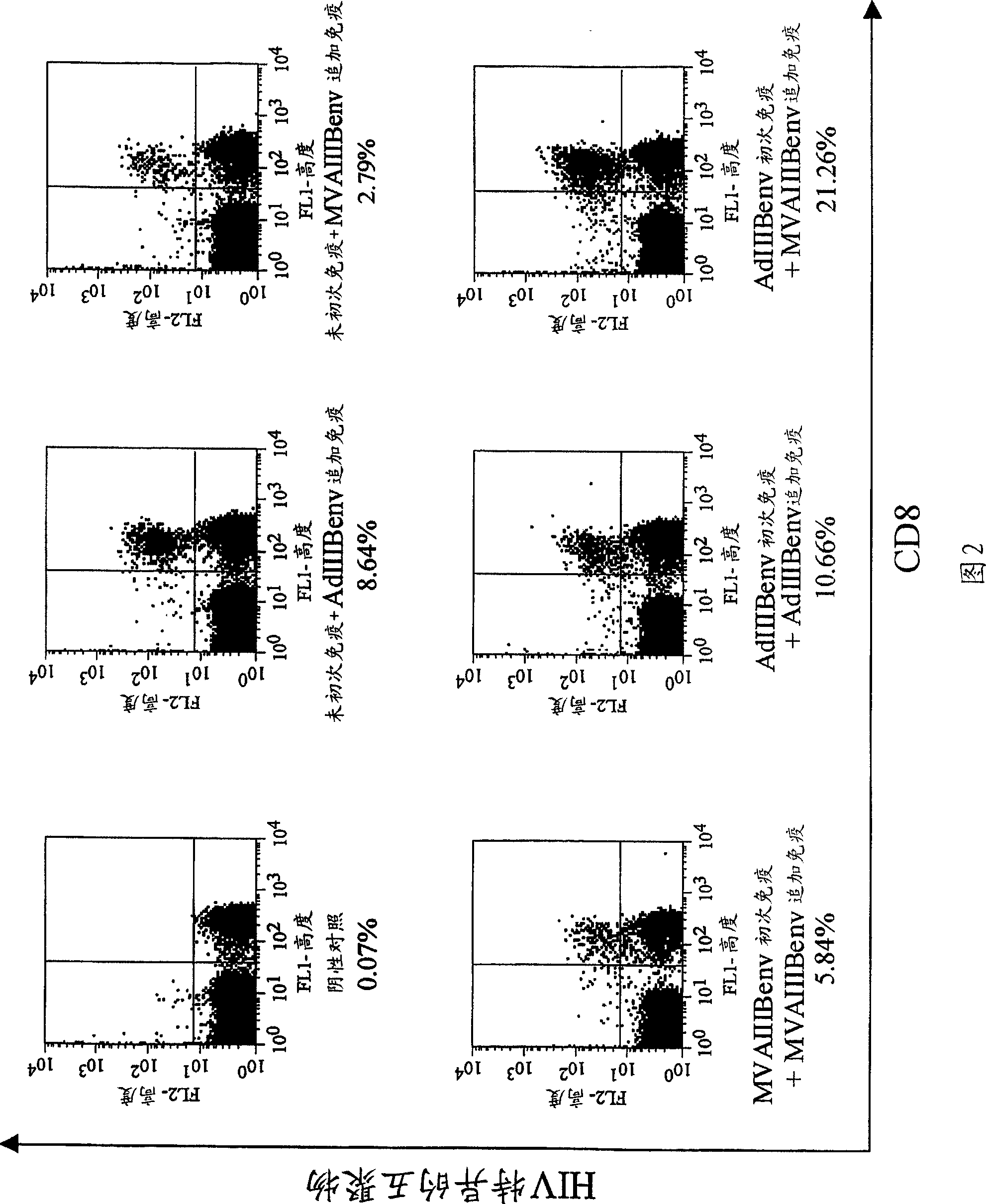
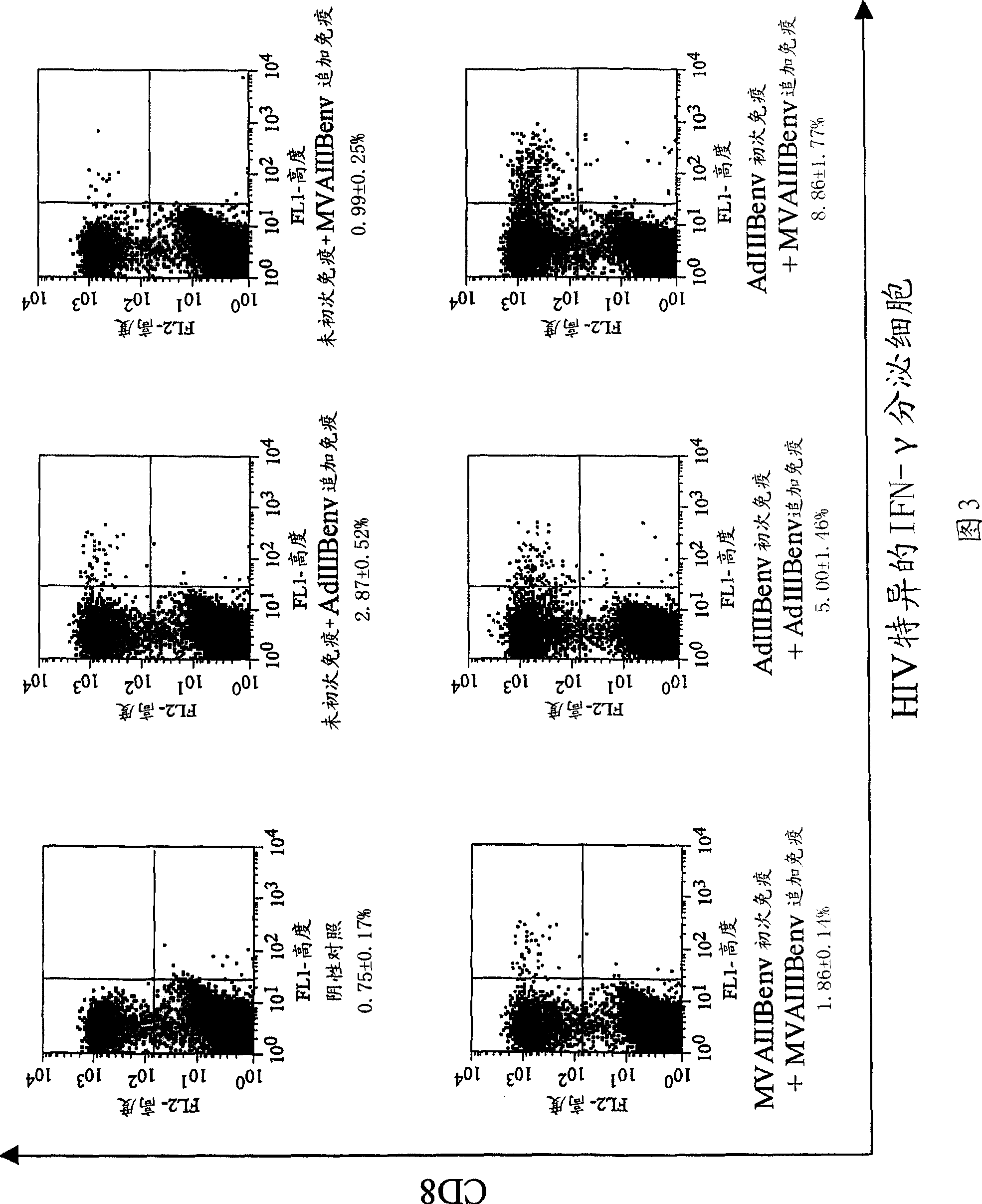
Strong immune induction by using combination of adenovirus type-5/type-35 vector and vaccinia virus mva vector
A technology of recombinant virus vector and adenovirus, which is applied in the field of anti-HIV drugs, can solve the problems of inability to produce strong immune induction, inability to administer large amounts of drugs, and strong liver toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Experimental methods and materials
[0076] recombinant vector
[0077] The recombinant virus obtained by substituting the cilia of non-propagating adenovirus type 5 lacking E1 and E3 with the cilia derived from adenovirus type 35, and introducing the rev gene and env gene of HIVIIIB, which is an HIV strain of subtype B, was used Ad generation kit (Avior Therapeutics Inc., Seattle, WA, USA) (Shayakhmetov D.M., et al., J. Virol. 2000; 74:2567-2583) was constructed. Simply stated, will contain the CAG promoter-HIV IIIB A 5.2k bp Sall / Pstl fragment of rev / envgp160-polyA was isolated from pCAGrev / env (Jounai N. et al., J. Gene Med. 2003; 5:609-617). The shuttle plasmid (pLHSP) containing the 22-342 site, 3523-5790 site of adenovirus type 5 (Ad5), E. coliori, and the ampicillin resistance gene was obtained from Avior Therapeutics Inc. (Seattle, WA, USA). The blunt-ended 5.2 kbp fragment was subcloned in the blunt-ended EcoRI site of pLHSP to generate the pLHSP-HIV shuttle...
Embodiment 2
[0103] recombinant vector
[0104]The preparation method of the MVA vaccine that reports SIVenv and SIVgag: Insert SIVenv (GenBankNo.AAA47632.1) and SIVgag genes (GenBank No.AAA47637.1) (Dr.ThomasC Friedrich, Wisconsin national Primate Research Center, Madison, WI53706 USA provides) To the SalI / PstI site of the MVA virus shuttle vector (pLW44) (provided by Dr. Bernard Moss, LVD, NIAID, NIH, Bethesda, MD 20892-0445 USA. PNAS101:6641-46, 2004). The successfully inserted vector was introduced into BHK21 cells (American Type culture Collection, The Global Bioresource Center, Manassas, VA20108, USA) infected with wild strain MVA (provided by Dr. Bernard Moss) with lipofbctamin (Invitrogen, Carisbad, CA92008, USA) . The recombinant MVA virus was refined with sucrose (Sucrose) (Wako, Tokyo, Japan). Report the making method of the Ad5 / 35 vector of SIVenv and SIVgag: use SIVenv (GenBank No.AAA47632.1) and SIVgag gene (GenBank No.AAA47637.1) (Dr.Thomas C Friedrich, Wisconsin national ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com