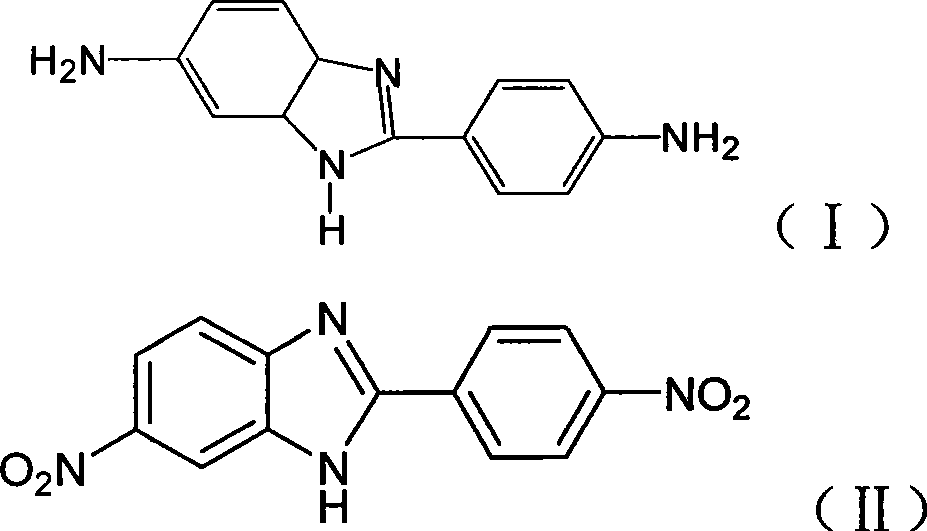
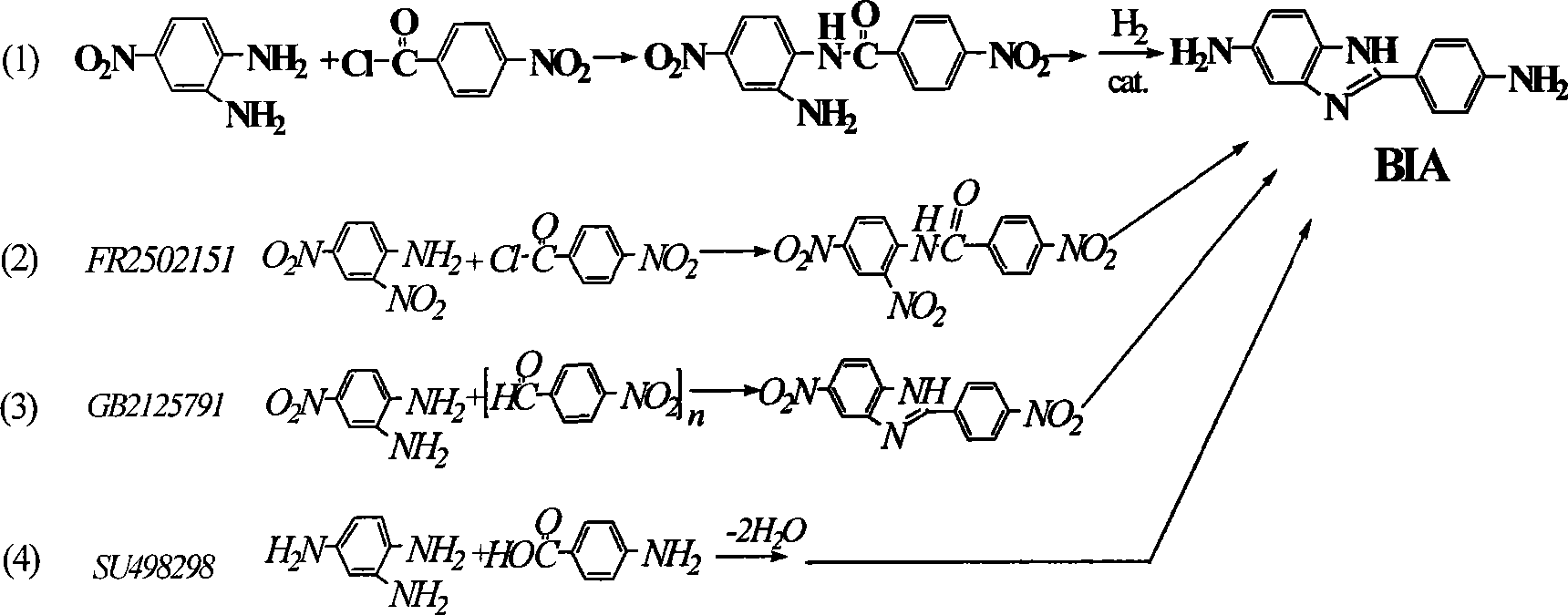
Method for preparing 2-( p-aminophenyl) benzimidazole-5-amine
A technology of p-aminophenyl and benzimidazole, which is applied in the field of preparation of 2-benzimidazol-5-amine, can solve problems such as difficulty in obtaining, difficulty in industrialization process, and great difficulty, and achieves short synthesis route and reduction technology Environmental protection and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
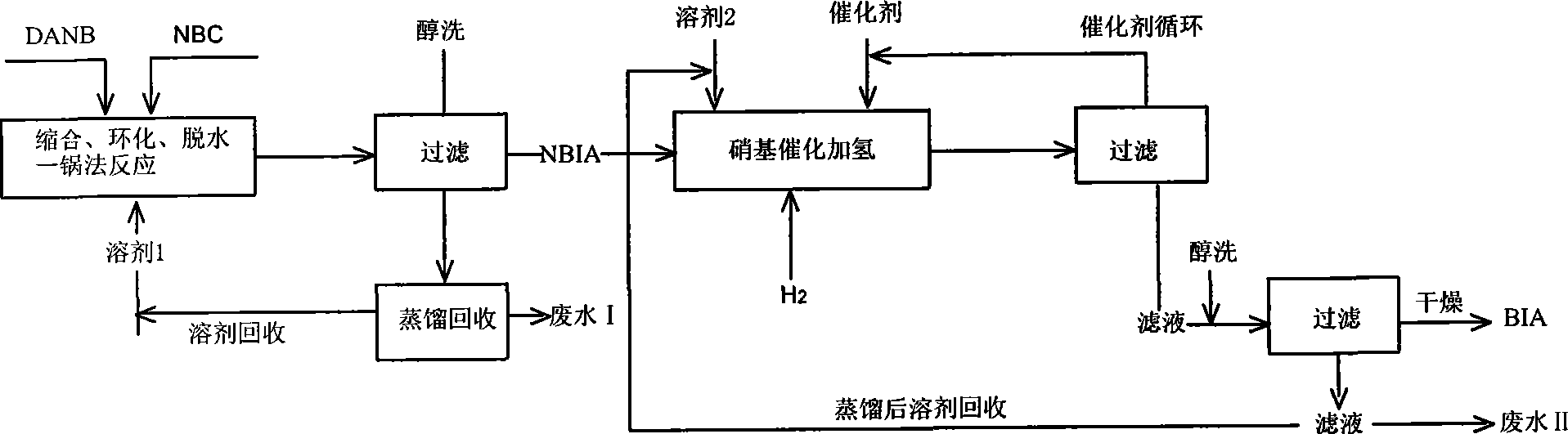
[0033] Add 150g of 1-methyl-2-pyrrolidone into a 500mL four-neck flask, add 25g of DANB and 32g of NBC under stirring, then raise the temperature to 185-190°C at 5°C / min, and keep the temperature constant for 3hr. After sampling, perform HPLC analysis to control the condensate content to <1.0%. After passing the analysis, cool the reaction solution to 100°C. Then slowly pour the reaction solution into 300g of normal temperature methanol prepared in advance, stir slowly until the product is precipitated, stop stirring, let stand to cool to room temperature, filter and drain. The filter cake was beaten with 300g of anhydrous methanol at room temperature for 30min, filtered and drained. Finally, the NBIA sample was obtained by circulating hot air at 60° C. for 24 hours. The HPLC purity was 98.86%, and the yield was 86.00%.
Embodiment 2
[0035] Add 150g of 1-methyl-2-pyrrolidone into a 500mL four-neck flask, add 25g of DANB and 32g of NBC under stirring, then raise the temperature to 130-135°C at 5°C / min, and keep the temperature constant for 3hrs. Then the reaction solution was cooled to 100°C and slowly poured into 300g of methanol at room temperature prepared in advance, stirred slowly until the product was precipitated, then stopped stirring, left to cool to room temperature, filtered and drained. The filter cake was beaten with 300g of anhydrous methanol at room temperature for 30min, filtered and drained. Finally, the NBIA sample was obtained by circulating hot air at 60° C. for 24 hours. The HPLC purity was 86.33%, and the yield was 25.08%.
Embodiment 3
[0037]Add 150g of 1-methyl-2-pyrrolidone into a 500mL four-neck flask, add 25g of DANB and 32g of NBC under stirring, then raise the temperature to 150-155°C at 5°C / min, and keep the temperature constant for 3hrs. Then the reaction solution was cooled to 100°C and slowly poured into 300g of methanol at room temperature prepared in advance, stirred slowly until the product was precipitated, then stopped stirring, left to cool to room temperature, filtered and drained. The filter cake was beaten with 300g of anhydrous methanol at room temperature for 30min, filtered and drained. Finally, the NBIA sample was obtained by circulating hot air at 60° C. for 24 hours. The HPLC purity was 95.89%, and the yield was 52.46%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com