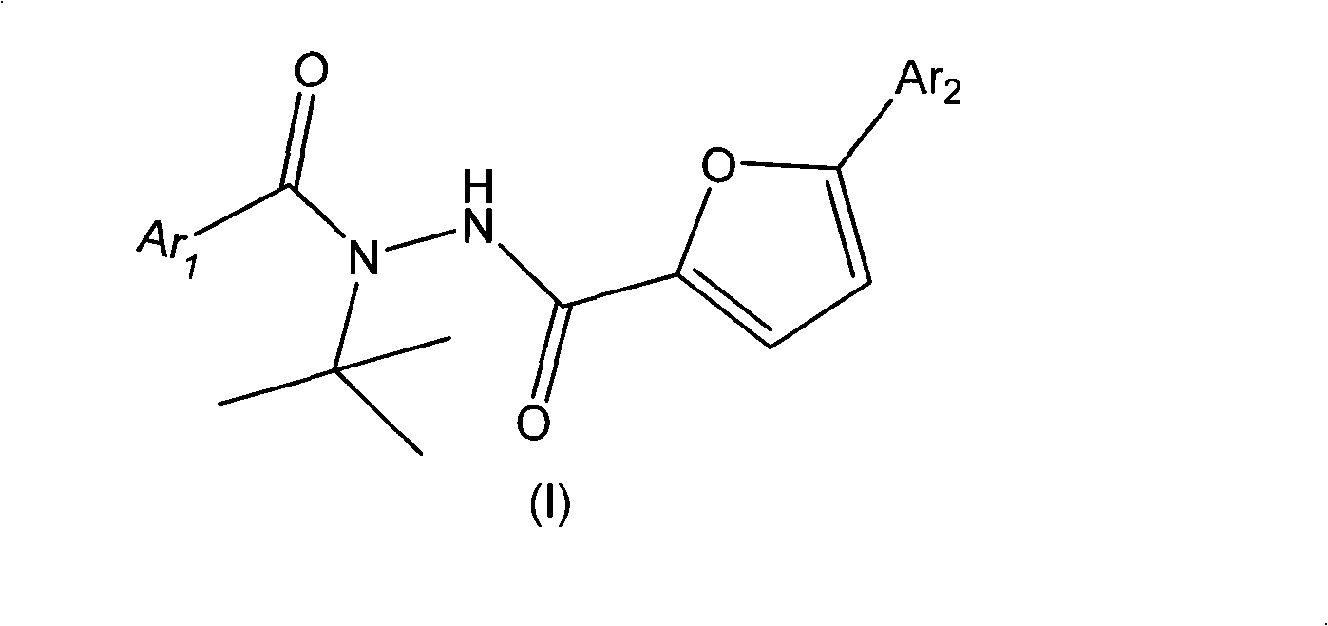
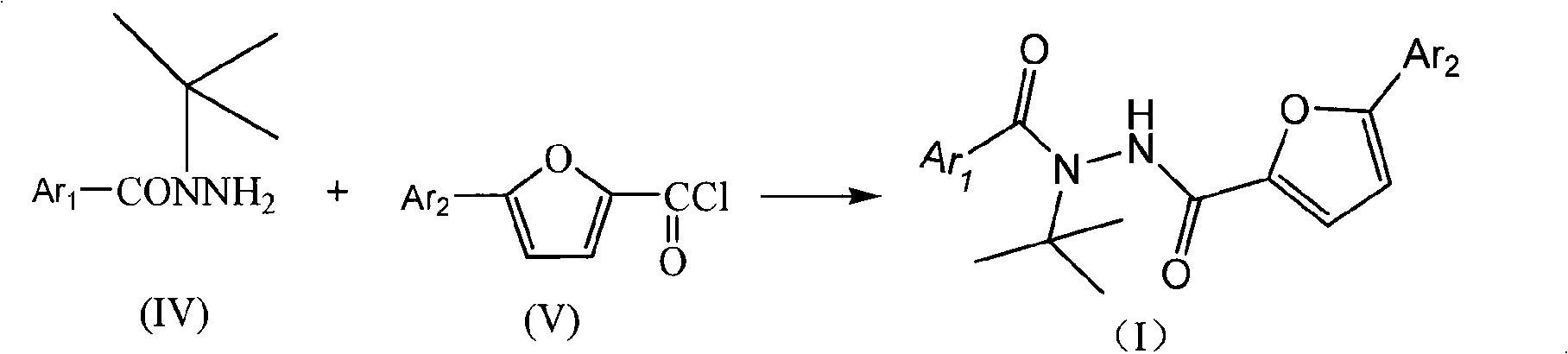
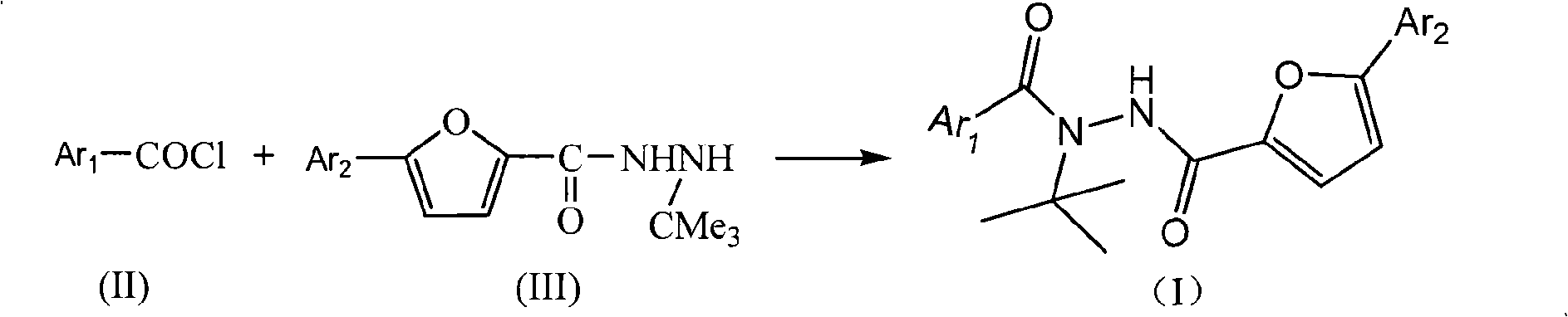Aryl hydrazide compounds with antineoplastic activity
A compound, aryl technology, applied in the application field of anti-tumor growth inhibitor, can solve the problem of unmentioned anti-tumor activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the preparation of p-chlorobenzoyl chloride
[0034]
[0035] Add 1.57g (0.01mol) of p-chlorobenzoic acid and 20ml of toluene into a 50ml three-necked flask, and add 3.57g (0.03mol) of thionyl chloride under stirring. Heating to reflux for 5 hours, distilling off the toluene and the remaining thionyl chloride, and then performing vacuum distillation to collect the fraction at 64~67°C / 0.67kPa to obtain 1.28g of colorless liquid with a yield of 73.6%.
Embodiment 2
[0036] Example 2: Preparation of N-tert-butyl-5-(4'-chloro-phenyl)furan-2-formylhydrazide
[0037]
[0038] Add 2g (0.016mol) of tert-butylhydrazine hydrochloride and 10ml of dichloromethane into a 50ml three-necked flask equipped with a thermometer, stir and cool to 0°C, add dropwise 6.4g (0.016mol) of 10% aqueous sodium hydroxide solution, Control the rate of addition to keep the temperature around 0°C. After the dropwise addition was completed, stir for 30 min. A solution of 0.96 g (0.004 mol) of p-chlorophenylfuroyl chloride in 10 ml of dichloromethane and 1.6 g (0.004 mol) of 10% aqueous sodium hydroxide solution were added dropwise at 0°C. Control the rate of addition so that both are added at the same time. After the dropwise addition, slowly rise to room temperature and react at 25°C for 5.5-6h. After filtering, the organic layer was washed with water and dried overnight with magnesium sulfate. After the desiccant was filtered off, the solvent was distilled off ...
Embodiment 3
[0039] Example 3: Preparation of N-tert-butyl-N'-(4'-chlorobenzoyl)-5-(4'-chlorophenyl)furan-2-formylhydrazide (I2)
[0040]
[0041] Add 1.46g (0.005mol) of N'-tert-butyl-5-(4'-chloro-phenyl)furan-2-carboxylhydrazide and 10ml of anhydrous toluene to a 50ml three-necked flask equipped with a thermometer. The temperature was lowered to 0°C, and at the same time, a solution of 0.875g (0.005mol) p-chlorobenzoyl chloride in 10ml of toluene and 0.5g of 40% NaOH aqueous solution were added dropwise, and the temperature was controlled at 0-5°C. After dripping, it was slowly raised to room temperature and reacted for 2h. Filtration gave a white solid, which was washed with ether-methanol (V乙醚 / V 甲醇 =1 / 1) to obtain 1.86 g of white needle-like crystals with a yield of 86.3% and a melting point of 221.5-223.5°C.
[0042] 1 H-NMR (DMSO-d 6 ,δ H ppm): 1.50(s, 9H, t-Bu), 7.11~7.12(d, 1H, FuH), 7.15~7.16(d, 1H, FuH), 7.36~7.39(d, 2H, ArH), 7.49~7.52 (d, 2H, ArH), 7.55-7.57 (d, 2H, A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com