A kind of synthetic method of methoxamine hydrochloride
A technology of methoxamine hydrochloride and a synthetic method, applied in the field of organic chemical synthesis, can solve problems such as limiting wide application, and achieve the effects of high yield, easy to scale up production, and simple conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
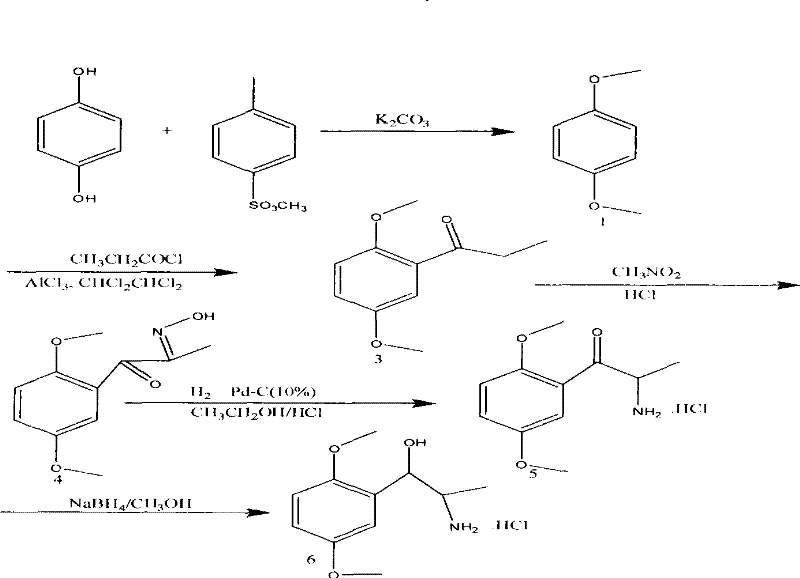
[0014] 1. Preparation of p-dimethoxybenzene: under nitrogen protection, add 33.6 grams of potassium carbonate and 90 grams of industrial acetonitrile in a 0.5-liter clean and water-free four-necked bottle, stir for 5 minutes, and then add 44.5 grams of liquid For methyl p-toluenesulfonate, wash the mouth of the bottle with 10 g of acetonitrile. Stir for 5 minutes, control the temperature to 15°C±3°C, and add 104.7g of acetonitrile mixture containing 16.7g of hydroquinone dropwise (about 1 hour). Stirring for another 5 minutes, the temperature was raised to 83° C. and the reaction was vigorously refluxed, and the nitrogen gas was turned off when reflux occurred. At this time, when gas is generated, the system becomes a pale yellow-white turbid liquid. TLC plate (ethyl acetate: dichloromethane = 1:30), until the raw material point (mung bean point) disappears, that is, the reaction is complete. After about 22 hours, the reaction was completed, and then the temperature was lowe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com