Nimesulide sustained-release tablet and preparation method thereof
A sustained-release tablet and compressibility technology, applied in the field of nimesulide sustained-release tablets, to achieve the effects of reducing surface tension, good process reproducibility, and reducing the number of times of taking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
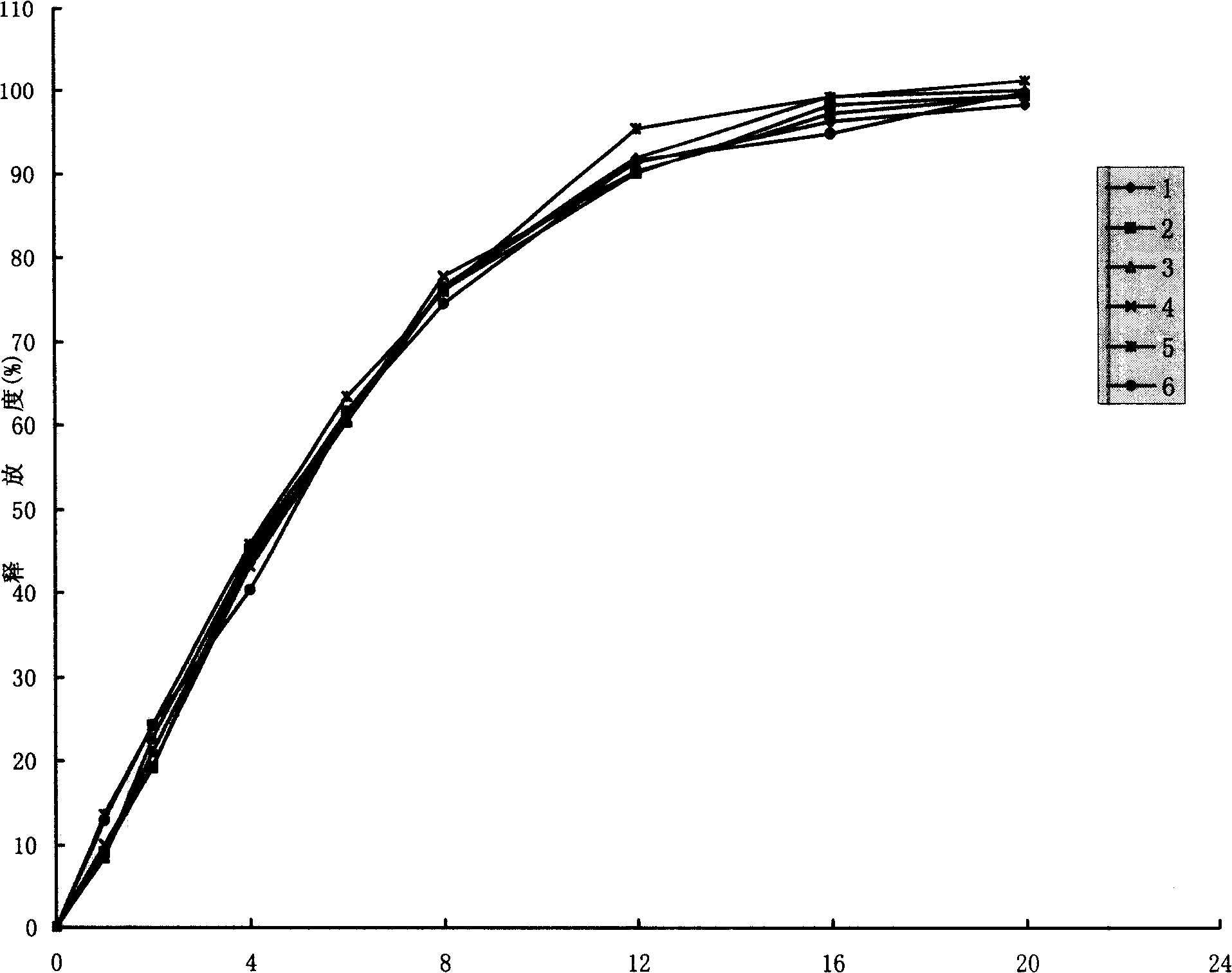
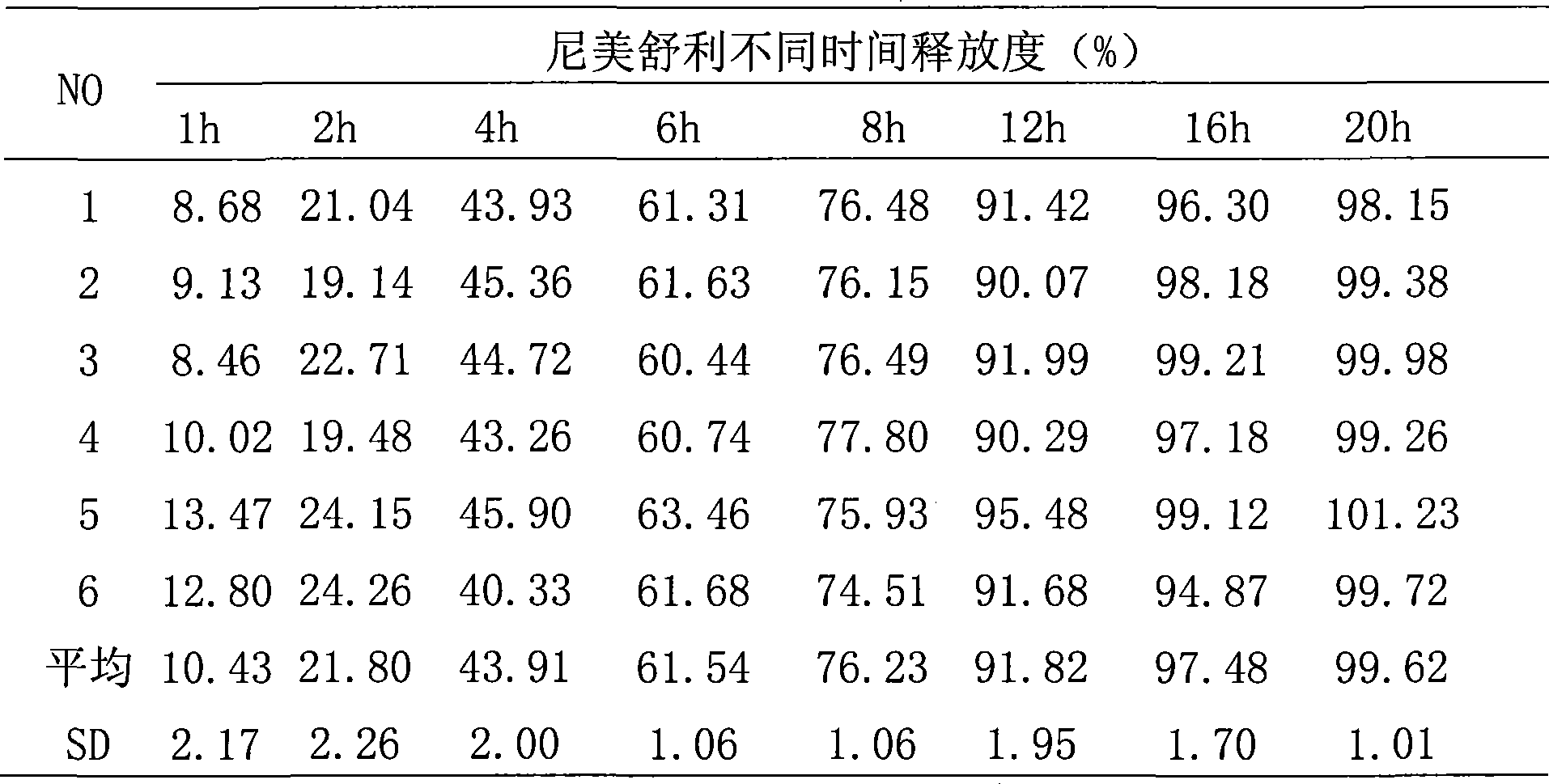
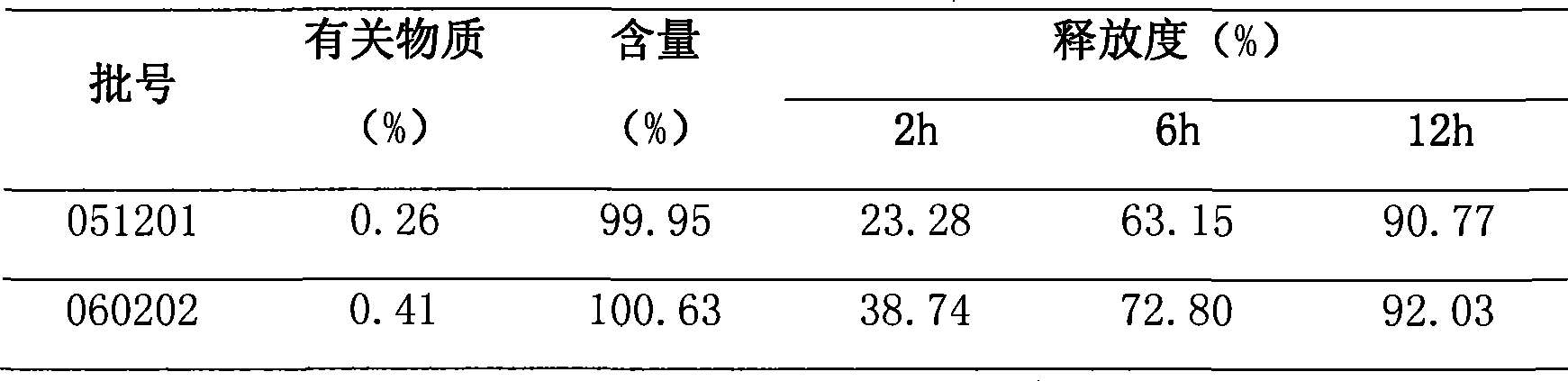
Image
Examples
Embodiment 1
[0021] 1000 Nimesulide Sustained Release Tablets, each containing Nimesulide 200mg.
[0022] Nimesulide 200g,
[0023] Hypromellose 60RT50 100g,
[0024] Compressible starch 100g,
[0025] Magnesium Stearate 5g,
[0026] 2.5% ethyl cellulose 95% ethanol solution 140ml.
[0027] Preparation method: mix nimesulide, hypromellose, compressible starch, etc. uniformly according to the method of equal amount addition, and use 140ml of 2.5% ethylcellulose and 95% ethanol solution to make the powder after mixing uniformly Material, pass through 16 mesh sieve to granulate, ventilate and dry at 55~60℃, control water content <3%, after granulation with 14 mesh sieve, add magnesium stearate in prescribed amount, mix well, compress into tablets, control tablet The hardness is between 6.5 and 7.5kg.
Embodiment 2
[0029] 1000 Nimesulide Sustained Release Tablets, each containing Nimesulide 200mg.
[0030] Nimesulide 200g,
[0031] High viscosity hypromellose 90g,
[0032] Low viscosity hypromellose 10g,
[0033] Compressible starch 100g,
[0034] Magnesium Stearate 5g,
[0035] 2.5% ethyl cellulose 95% ethanol solution 140ml.
[0036] Preparation method: mix high-viscosity hypromellose and low-viscosity hypromellose to form hypromellose with a viscosity of 4000 milliPascal seconds, and add nimesulide and hypromellose to , compressible starch, etc., mix well, use 140ml of 2.5% ethyl cellulose and 95% ethanol solution to make the above mixed powder into a soft material, pass through a 16-mesh sieve to granulate, and ventilate and dry at 55-60°C to control the content of The amount of water is less than 3%. After the granules are sized with a 14-mesh sieve, the prescribed amount of magnesium stearate is added, mixed evenly, and compressed into tablets. The hardness of the tablets is c...
Embodiment 3
[0038] 1000 Nimesulide Sustained Release Tablets, each containing Nimesulide 200mg.
[0039] Nimesulide 200g,
[0040] High viscosity hypromellose 65g,
[0041] Low viscosity hypromellose 35g,
[0042] Compressible starch 100g,
[0043] Magnesium Stearate 5g,
[0044] 4.5% polyvinylpyrrolidone 95% ethanol solution 120ml.
[0045] The preparation method is the same as in Example 2, wherein the high viscosity hypromellose and the low viscosity hypromellose are mixed to form the hypromellose with a viscosity range of 2000 milliPascals.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com