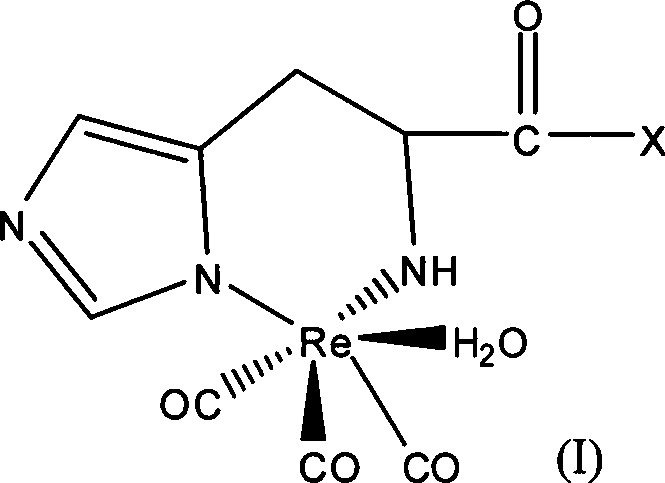
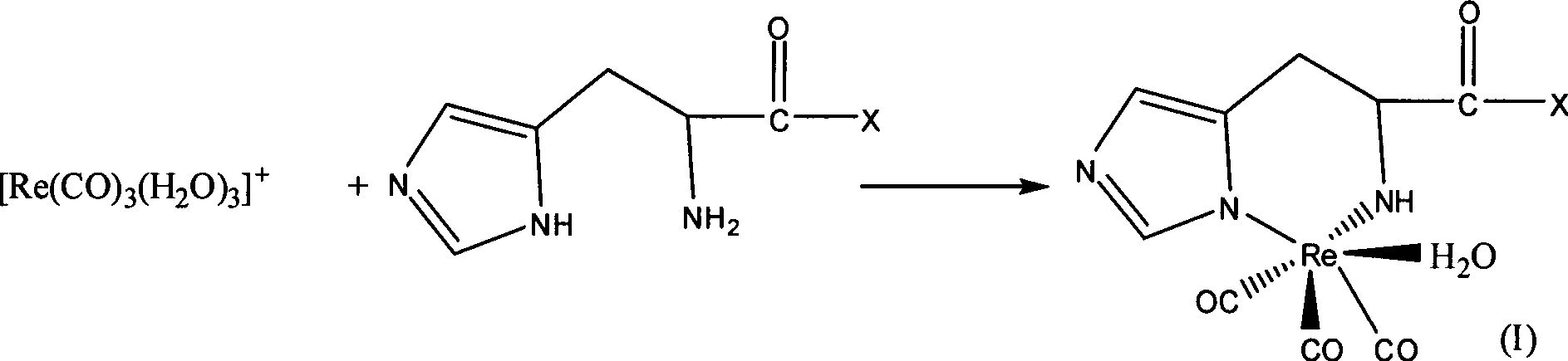
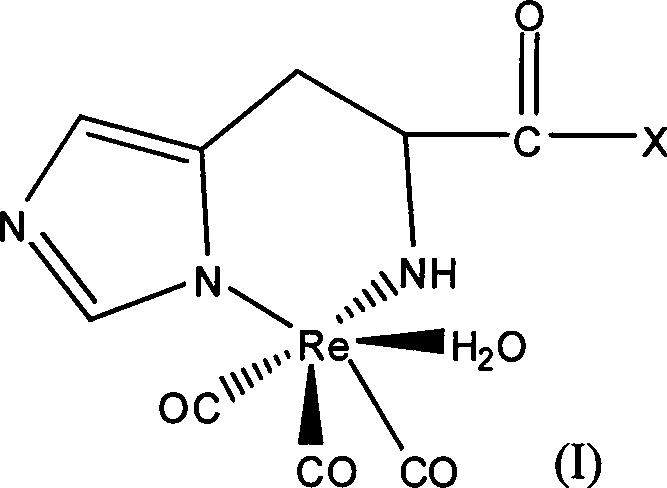
Radioactive rhenium marked polypeptide containing RGD sequence as well as preparation method and application thereof
A radioactivity and sequence technology, applied in the field of peptides, can solve problems such as affecting the activity of peptides, and achieve the effects of high labeling rate, simple operation, and easy availability of raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Preparation of Compound 1
[0024] 1. According to the prior art, fac-[ 188 Re(CO) 3 (H 2 O) 3 ] + Synthesis and identification of
[0025] Weigh 4.5mg BH 3 .NH 3 (chemical name borane ammonium complex) is added in a clean and dry 10ml vial, and sealed with a cap. Pass CO gas into the vial for about 20 minutes. 8 μl of phosphoric acid with a concentration > 85% and 1 ml of Na with a radioactivity of 3 mCi / ml 188 ReO 4The normal saline eluent was mixed, and then injected into the vial that had been filled with CO gas in advance. React in a 70°C water bath for 15 minutes. Immediately after the reaction, it was cooled with ice water. The reaction mixture was filtered through a QMA Spe-Pak column for separation and purification. The resulting fac-[ 188 Re(CO) 3 (H 2 O) 3 ] + The radioactive purity reaches more than 95%.
[0026] The reaction products were identified by thin-plate chromatography (TLC) and high-performance liquid chromatography (H...
Embodiment 2
[0034] Example 2 Preparation of Compound 2
[0035] fac-[ 188 Re(CO) 3 (H 2 O) 3 ] + The synthesis is the same as in Example 1. Take 50μl concentration as 10 -4 mol of polypeptide b (its structure is shown in Table 1, synthesized by Shanghai Jier Biological Co., Ltd.) and 450 μl of fac-[ 188 Re(CO) 3 (H 2 O) 3 ] + Mix well. React at 65°C for 20 minutes to obtain the corresponding complex (namely compound 2). The same HPLC and double developing agent TLC as in Example 1 were used to determine the labeling rate of the reaction. For HPLC purification of peptides and 188 The Re-RGD retention times are summarized in Table 2. The marking rate is 93%.
Embodiment 3
[0036] Example 3 Preparation of compound 3
[0037] fac-[ 188 Re(CO) 3 (H 2 O) 3 ] + The synthesis is the same as in Example 1. Take 50μl concentration as 10 -4 mol / ml of polypeptide c (its structure is shown in Table 1, synthesized by Shanghai Jier Biological Co., Ltd.) and 450 μl of fac-[ 188 Re(CO) 3 (H 2 O) 3 ] + Mix well. React at 65°C for 20 minutes to obtain the corresponding complex (namely compound 3). The same HPLC and double developing agent TLC as in Example 1 were used to determine the labeling rate of the reaction. For HPLC purification of peptides and 188 The Re-RGD retention times are summarized in Table 2. The marking rate is 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com