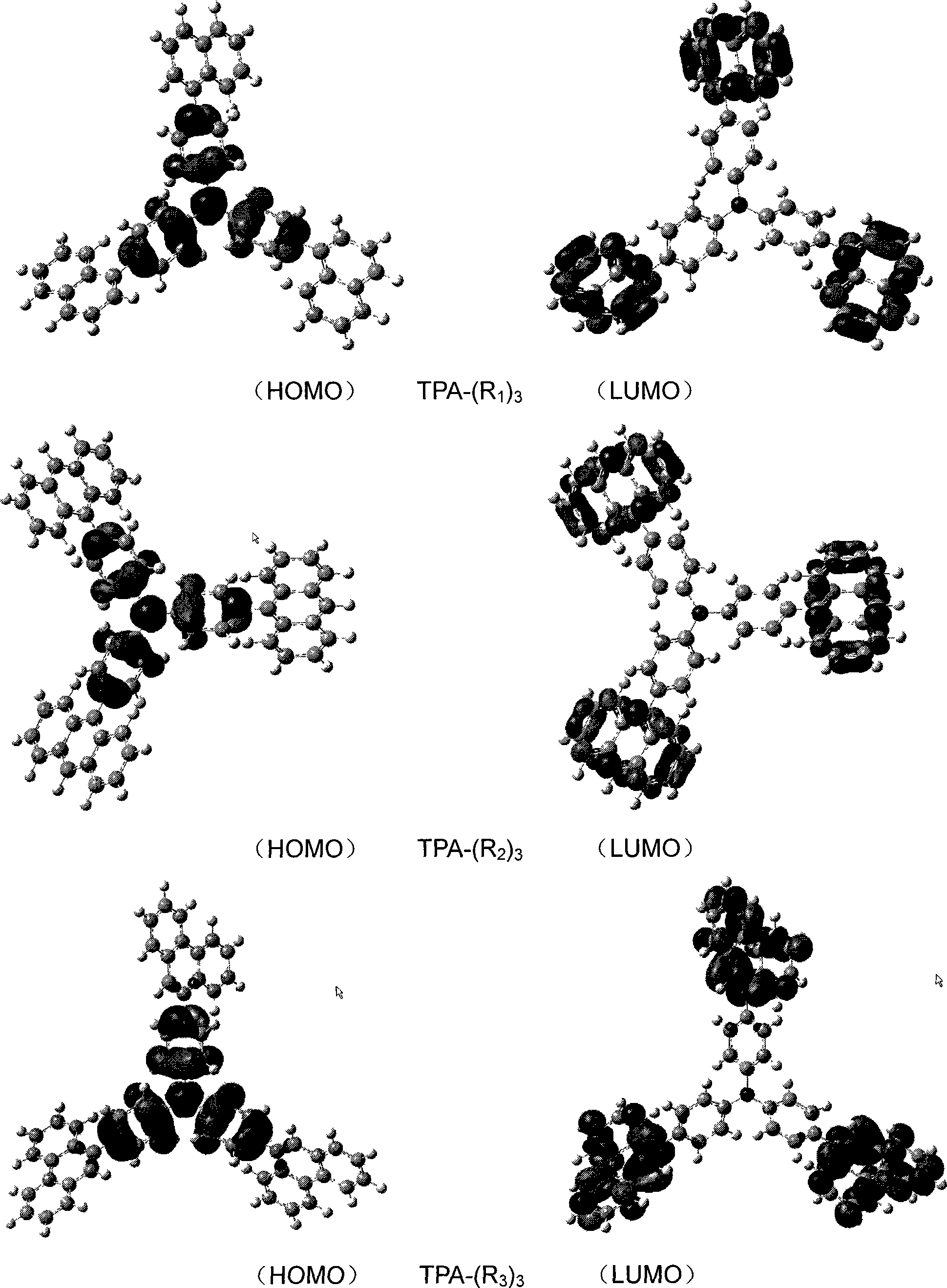
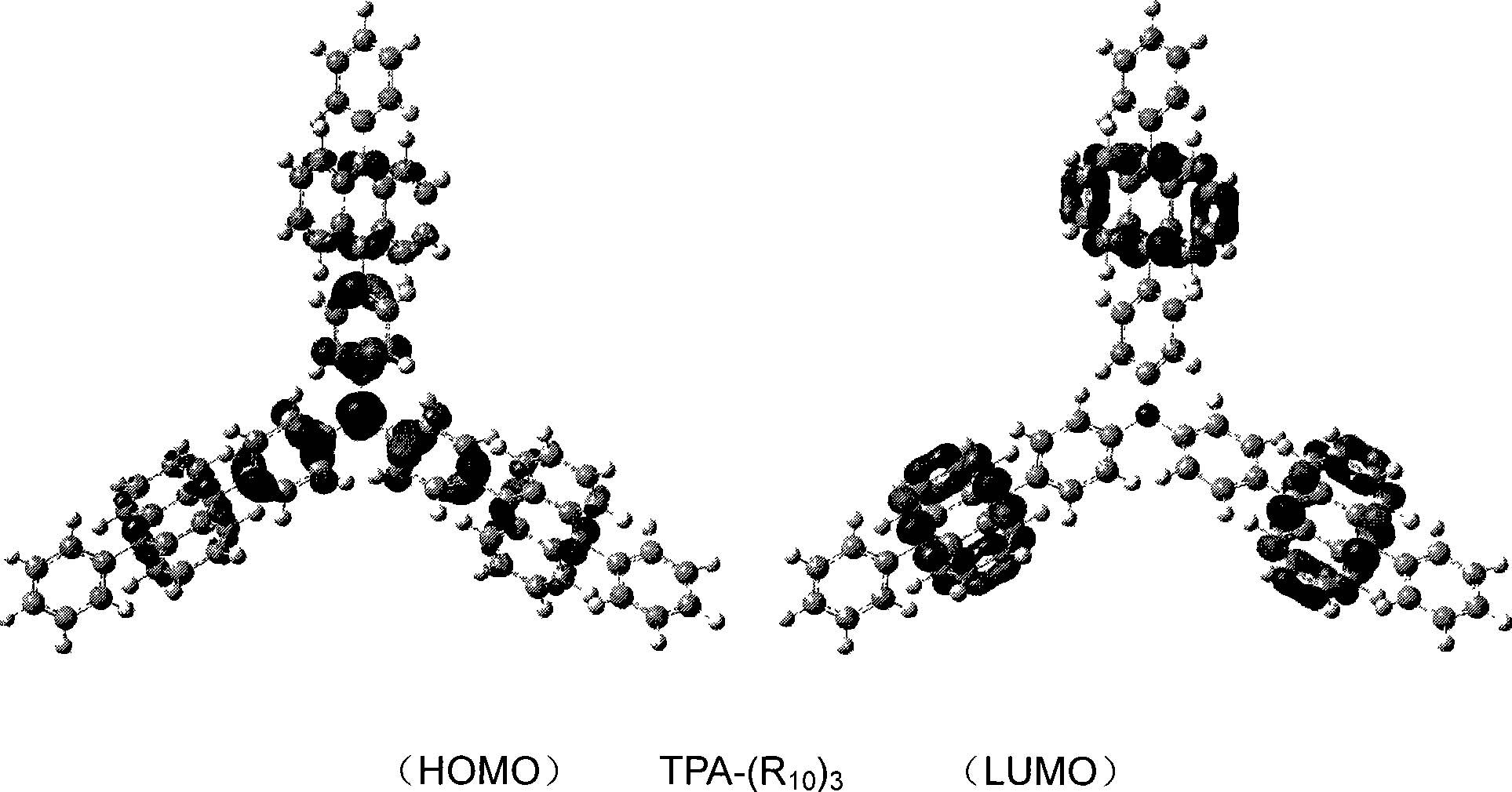
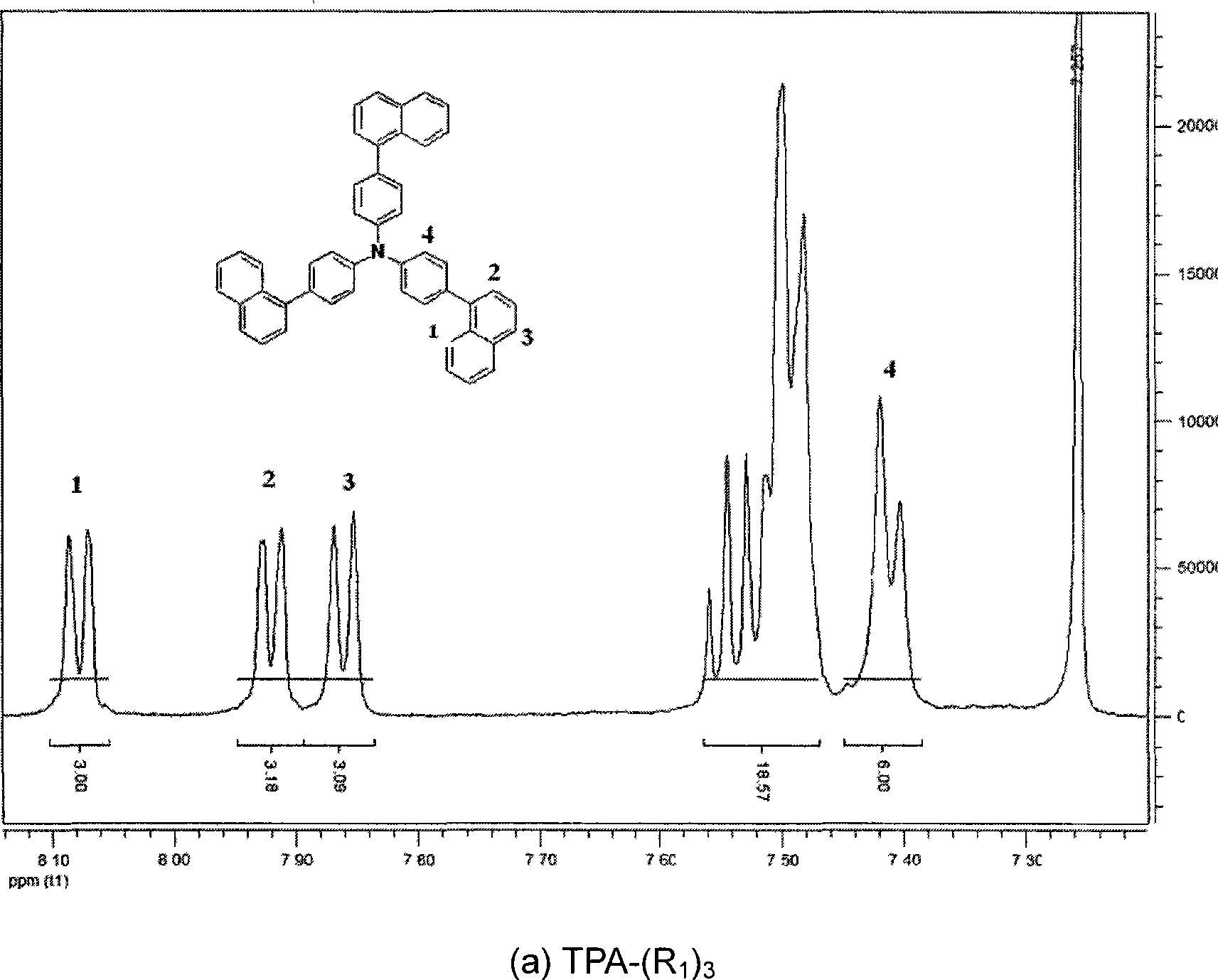
High performance electroluminescent organic material and application thereof in organic EL device
An electroluminescent material and high-performance technology, which is applied in the direction of luminescent materials, electric solid devices, electrical components, etc., can solve the problems of reducing fluorescence quenching efficiency, and achieve improved device stability, high efficiency, and high electroluminescent efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Embodiment 1: TPA-(R 1 ) 3 Synthesis
[0073] Compound 1: Synthesis of 9-(4,4,5,5-tetramethyl-1,3,2-dioxaborinane)-naphthalene In 50 ml of refined tetrahydrofuran (THF), place it in a low-temperature reactor at -78°C, slowly inject 4.1 ml of n-BuLi (2.5 mol / L), and inject 5.0 ml of boroester one hour later, After half an hour, it was removed from room temperature and stirred for 2 days. A light earth red turbid liquid was obtained. Wash with water, then extract with ether for 3-4 times, combine the ether solutions, and dry over anhydrous magnesium sulfate overnight. The desiccant was filtered, the filtrate was spin-dried, and then the column was run with petroleum ether:dichloromethane (4:1) as the eluent to isolate the pure target product. Yield: 52%.
[0074] Compound 2: Synthesis of Tris(4-bromophenyl)amine
[0075] Weigh 8.0 grams of triphenylamine, add about 60 milliliters of chloroform to dissolve, and slowly add 5.2 milliliters of liquid bromine (Br 2 ) a...
Embodiment 2
[0079] Embodiment 2: TPA-(R 2 ) 3 Synthesis
[0080] Compound 3: Synthesis of 9-(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)-anthracene
[0081] Weigh 2.57 grams of monobromoanthracene, dissolve it in about 120 milliliters of refined tetrahydrofuran (THF), place it in a low-temperature reactor to -78°C, and slowly inject 4.1 milliliters of n-butyllithium (n-BuLi) (2.5 mol / liter), inject 5.0 ml of boron ester one hour later, take it out after half an hour and stir at room temperature for 2 days. A dark orange-red cloudy solution was obtained. Wash with water, then extract with ether 3-4 times, combine the ether solution, anhydrous magnesium sulfate (MgSO 4 ) to dry overnight. The desiccant was filtered, the filtrate was spin-dried, and then the column was run with petroleum ether:dichloromethane (5:2) as the eluent to isolate the pure target product. Yield: 63%.
[0082] TPA-(R 2 ) 3 : Synthesis of Tris(4-(9-Anthracenyl)phenyl)amine
[0083] Weigh 216.9 mg of tris(4-bro...
Embodiment 3
[0085] Embodiment 3: TPA-(R 3 ) 3 Synthesis
[0086] Compound 4: Synthesis of 9-(4,4,5,5-tetramethyl-1,3,2-dioxaborinane)-phenanthrene
[0087] Weigh 2.0 g of monobromophenanthrene, dissolve it in about 100 ml of refined tetrahydrofuran (THF), place it in a low-temperature reactor to -78°C, and slowly inject 3.2 ml of n-butyllithium (2.5 mol / L) for one hour Then inject 5.0 ml of boron ester, take it out after half an hour and stir at room temperature for 2 days. A light green cloudy liquid was obtained. Wash with water, then extract with ether for 3-4 times, combine the ether solutions, and dry over anhydrous magnesium sulfate overnight. The desiccant was filtered, the filtrate was spin-dried, and then the column was run with petroleum ether:dichloromethane (5:2) as the eluent, and the pure target product was isolated. Yield: 26%.
[0088] TPA-(R 3 ) 3 : Synthesis of three (4-(9-phenanthrenyl) phenyl) amine
[0089] Weigh 216.9 mg of tris(4-bromophenyl)amine and 547.2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com