Nano lycorine chloride microcapsule latex and preparation method thereof
A technology of lycorine hydrochloride and nano-microcapsules, which is applied in the fields of emulsion delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve problems such as slow release, and achieve the effect of reducing strong effects and prolonging release time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
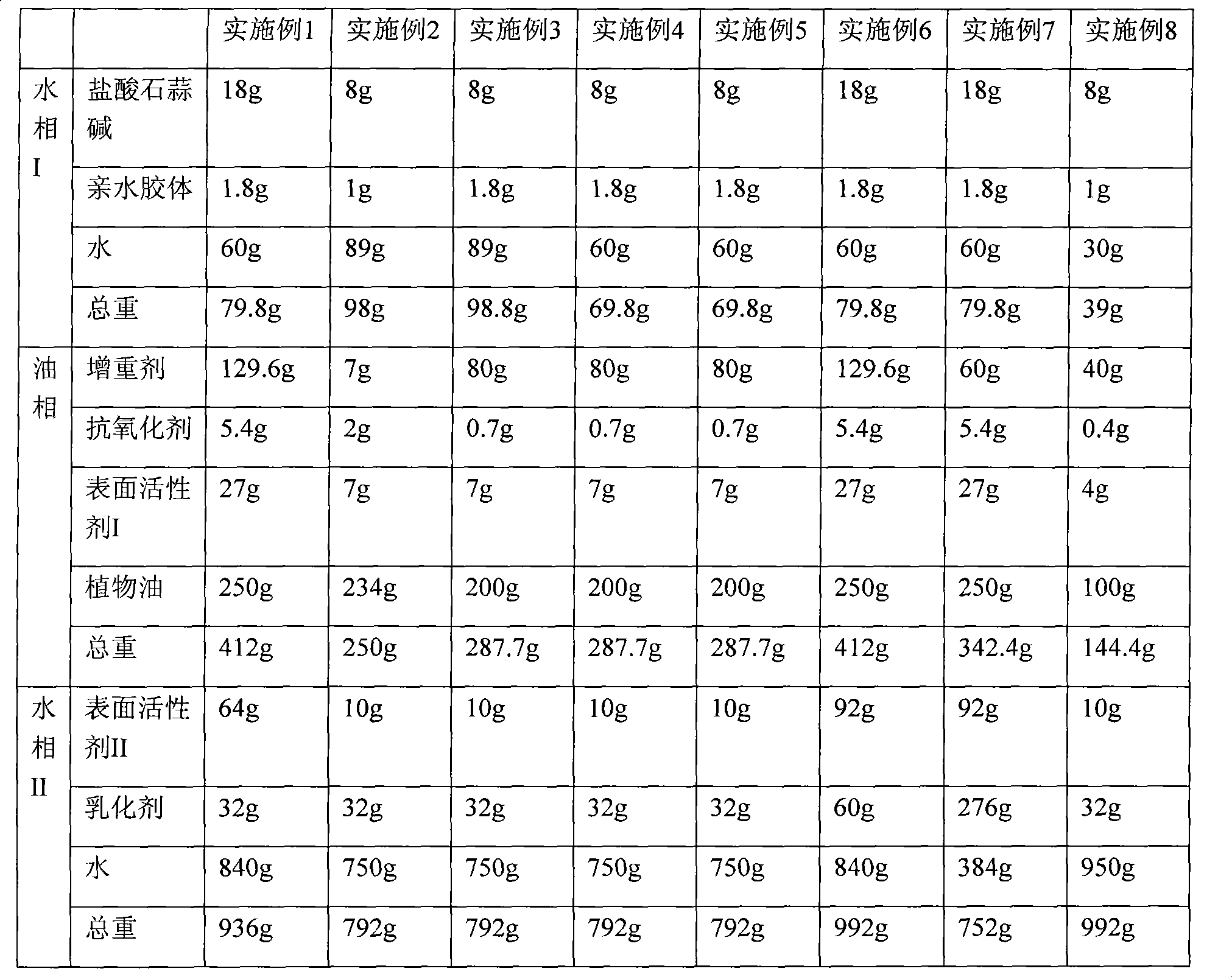
[0032] Weigh 18 grams of lycorine hydrochloride and 1.8 grams of gellan gum, and add them to 60 grams of water under stirring (stirring rate 50 rpm), until the lycorine hydrochloride and gellan gum are completely dissolved in water to obtain the water phase I.
[0033] Take by weighing 129.6 gram of weighting agent sucrose acetate isobutyrate (SAIB, U.S. Eastman company), 5.4 gram of antioxidant BHT, 27 gram of surfactant I Span60 (UK Croda company), under stirring situation (stirring speed 100rpm), Disperse and dissolve in 250 g of olive oil to obtain an oily phase.
[0034] Add the water phase I to the oil phase under high shear (10000rmp) to form a pre-emulsion.
[0035] Weigh 64 grams of surfactant II Tween 40 (Croda, UK), 32 grams of emulsifier gum arabic, add to 840 grams of water, stir and disperse evenly (stirring speed 50 rpm), to obtain water phase II.
[0036] The pre-emulsion was added to the aqueous phase II under stirring (stirring rate 50 rpm) to obtain a pre-m...
Embodiment 2
[0038] Weigh 8 grams of lycorine hydrochloride and 1 gram of gellan gum, and add them to 89 grams of water under stirring (stirring rate 100 rpm), until the lycorine hydrochloride and gellan gum are completely dissolved in water to obtain the water phase I.
[0039] Take by weighing 7 gram weight-increasing agent SAIB (U.S. Eastman company), 2 gram antioxidant BHA, 7 gram surfactant I Span80 (UK Croda company), under stirring situation (stirring speed 50rpm), disperse and dissolve in 234 gram palm oil , an oily phase was obtained.
[0040] Add the water phase I to the oil phase under high shear (5000rmp) to form a pre-emulsion.
[0041] Weigh 10 grams of surfactant II potassium oleate and 32 grams of emulsifier Purity Gum 1773 (National Starch Co.) into 750 grams of water, stir and disperse evenly (stirring speed 200 rpm), to obtain aqueous phase II.
[0042]The pre-emulsion was added to the aqueous phase II under stirring (stirring rate 1000 rpm) to obtain a pre-mixture. In...
Embodiment 3
[0044] Take by weighing 8 grams of lycorine hydrochloride, 0.8 gram of xanthan gum and 1 gram of gellan gum, and under stirring (stirring speed 500 rpm), join in 89 grams of water until lycorine hydrochloride and gellan gum are completely dissolved in water , to obtain the aqueous phase I.
[0045] Take by weighing 80 gram weight-increasing agent SAIB (U.S. Eastman company), 0.7 gram antioxidant BHA, 7 gram surfactant I Span85 (UK Croda company), under stirring situation (stirring rate 1000rpm), disperse and dissolve in 200 gram sunflower seeds oil to obtain an oily phase.
[0046] Add the water phase I to the oil phase under high shear (20000rmp) to form a pre-emulsion.
[0047] Take by weighing 10 gram surfactant II brij-35 (U.S. Amresco company), 22 gram emulsifier Purity Gum 1773 (National Starch Co.), 10 gram emulsifier guar gums join in 750 gram water, stir and disperse evenly (stirring speed 500rpm), to obtain the aqueous phase II.
[0048] The pre-emulsion was added...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com