Method for preparing medical lycorine emulsion
A technology of lycorine and emulsion, which is applied in the directions of non-active ingredient medical preparations, active ingredients-containing medical preparations, pharmaceutical formulas, etc., can solve the problems of slow release and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
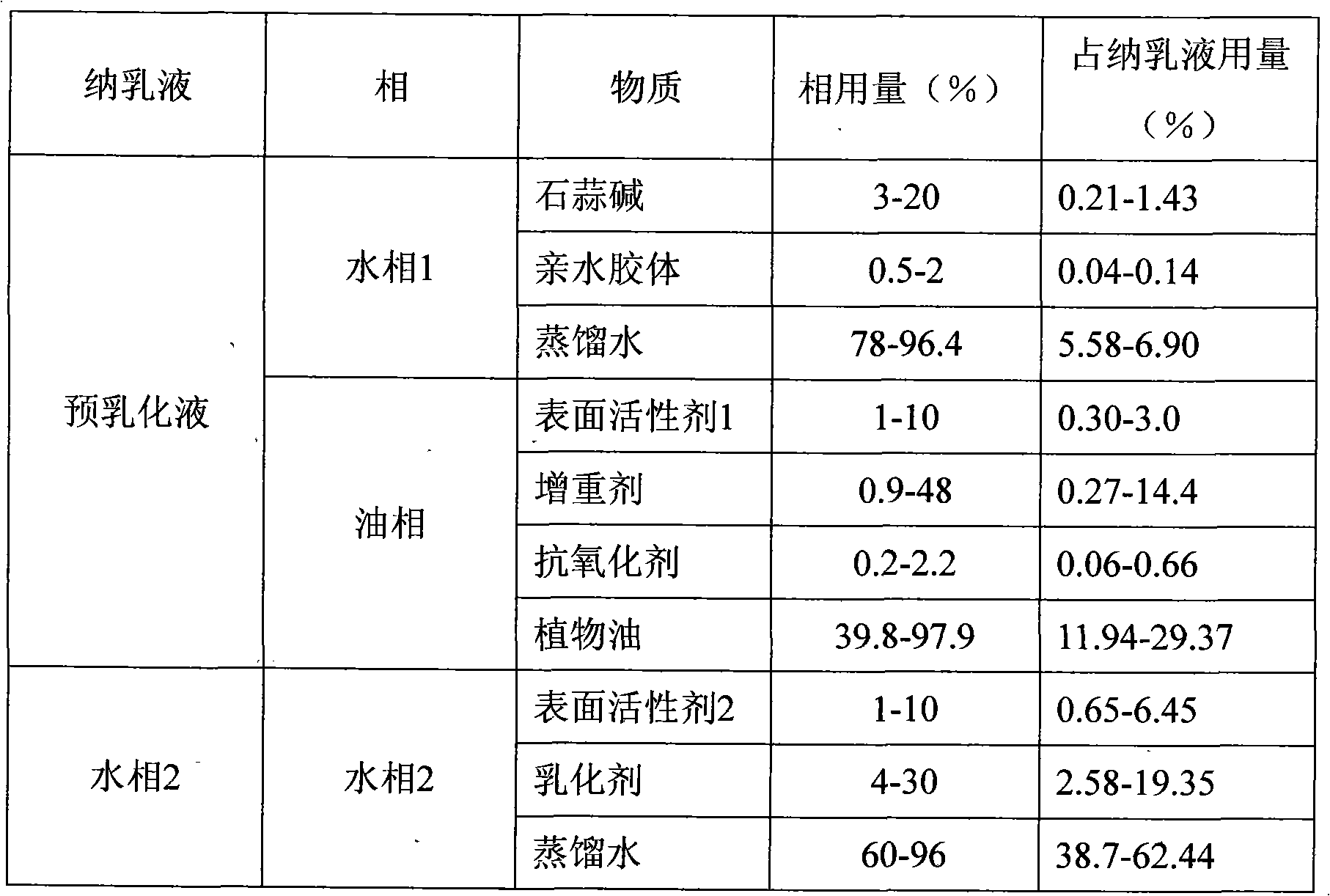
[0020] (1) Weigh 16 grams of lycorine and 1.6 grams of xanthan gum, and add them into 60 grams of distilled water while stirring, until the lycorine and xanthan gum are completely dissolved in the distilled water to obtain the water phase 1.
[0021] (2) Weigh 118.3 grams of weighting agent SAIB, 4.4 grams of antioxidant BHT, and 28 grams of surfactant Span60, and disperse and dissolve them in 250 grams of soybean oil under stirring to obtain an oil phase.
[0022] (3) Add the water phase 1 into the oil phase under high-speed shear (10000 rpm) to form a pre-emulsion.
[0023] (4) Weigh 8 grams of surfactant Myrij-45 and 32 grams of emulsifier gum arabic into 820 grams of distilled water, stir and disperse evenly to obtain water phase 2.
[0024] (5) under stirring, add the pre-emulsion to the water phase 2 to obtain the pre-mixture;
[0025] (6) Inject the pre-mixed liquid into the M-110EH high-pressure micro-fluidic nano-dispersion equipment, and homogenize twice under the p...
Embodiment 2
[0028] (1) Weigh 8 grams of lycorine and 0.8 grams of carrageenan, and add them to 92 grams of distilled water while stirring, until the lycorine and carrageenan are completely dissolved in the distilled water to obtain the water phase 1.
[0029] (2) Weigh 8 grams of weighting agent EG, 20 grams of antioxidant BHA, and 7 grams of surfactant Span85, and disperse and dissolve them in 250 grams of salad oil under stirring to obtain an oil phase.
[0030] (3) Add the water phase 1 into the oil phase under high-speed shear (5000 rpm) to form a pre-emulsion.
[0031] (4) Weigh 10 grams of surfactant brij-30 and 32 grams of emulsifier Purity Gum 1773 (manufactured by National Starch & Chemical Co.) into 750 grams of distilled water, stir and disperse evenly to obtain water phase 2.
[0032] (5) under stirring, add the pre-emulsion to the water phase 2 to obtain the pre-mixture;
[0033] (6) Inject the pre-mixed liquid into the M-110EH high-pressure micro-fluidic nano-dispersion equ...
Embodiment 3
[0035] (1) Weigh 8 grams of lycorine and 1 gram of guar gum, and add them into 92 grams of distilled water while stirring until the lycorine and guar gum are completely dissolved in the distilled water to obtain the water phase 1.
[0036] (2) Weigh 80 grams of weighting agent SAIB, 0.7 grams of antioxidant BHA, and 7 grams of surfactant Span80, and disperse and dissolve them in 200 grams of olive oil under stirring to obtain an oil phase.
[0037] (3) Add the water phase 1 into the oil phase under high-speed shear (20000 rpm) to form a pre-emulsion.
[0038] (4) Take by weighing 10 grams of surfactant Myrij-45, 22 grams of emulsifier Purity Gum 1773 (produced by Nationalstarch & chemical Co), 10 grams of emulsifier guar gum and join in 750 grams of distilled water, stir and disperse evenly to obtain water phase 2 .
[0039] (5) under stirring, add the pre-emulsion to the water phase 2 to obtain the pre-mixture;
[0040] (6) Inject the pre-mixed liquid into the M-110EH high-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com