Method for deoxidizing solid catalyst precursor
A solid catalyst and precursor technology, applied in chemical instruments and methods, catalyst activation/preparation, physical/chemical process catalysts, etc., can solve the problems of reducing the surface area of particles, reducing the number of active sites of catalysts, and difficulty in reducing metal oxides. Achieve the effect of reducing heat energy consumption, inhibiting migration and agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
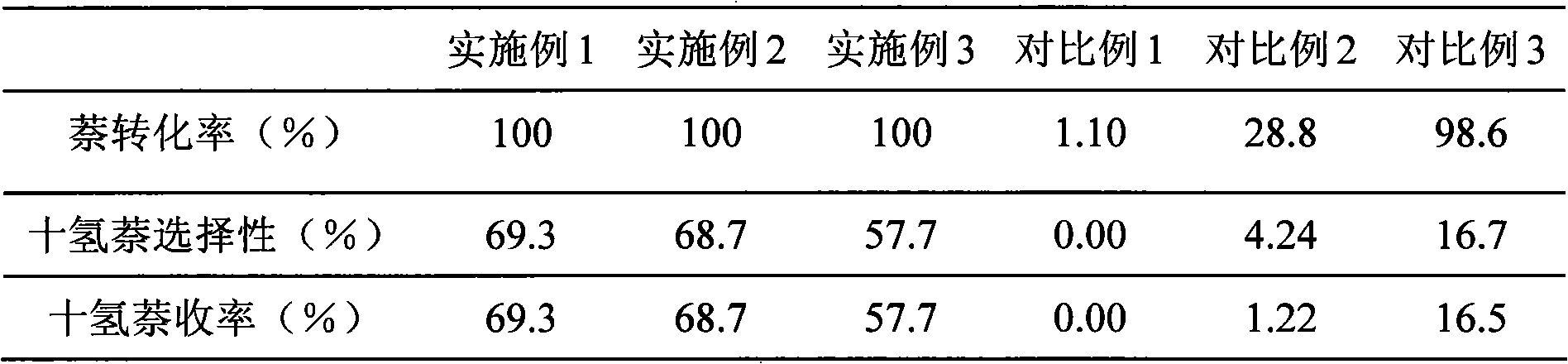
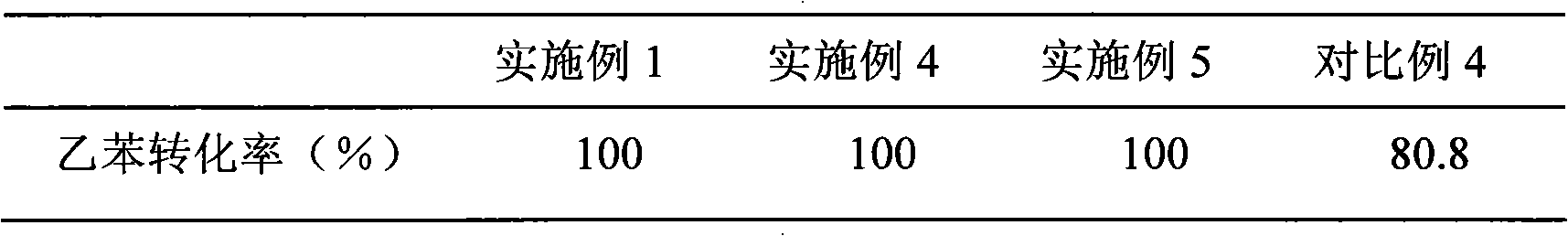
Embodiment 1
[0015] Weigh 18.5g of nickel acetate and add it into 50ml of water, fully stir and dissolve with a magnetic stirrer at 40°C, then add 15g of γ-Al 2 o 3 Carrier, continue to stir, pour out the clear liquid after 5 hours, dry at 150°C for 8 hours, sieve the catalyst into 40-60 mesh particles, and put it into a stainless steel reaction tube with an inner diameter of 12mm. At room temperature, hydrogen gas was introduced at a flow rate of 27mL / min. After the air was exhausted, the reaction tube was directly placed in a heating furnace at 800°C, and the catalyst was reduced for 10 minutes. Finally, the reaction tube was directly removed from the heating furnace without turning off the reducing gas. Take it out and place it at room temperature.
[0016] Ni / γ-Al obtained by reduction 2 o 3 Catalyst samples were evaluated in an autoclave for their naphthalene hydrogenation activity, which was characterized by the yield of decahydronaphthalene, which was defined as the product of th...
Embodiment 2
[0019] Weigh 18.5g of nickel acetate and add it into 50g of water, fully stir and dissolve with a magnetic stirrer at 40°C, then add 15g of γ-Al 2 o 3 Carrier, continue to stir, pour out the clear liquid after 5 hours, dry at 150°C for 8 hours, sieve the catalyst into 40-60 mesh particles, and put it into a stainless steel reaction tube with an inner diameter of 12mm. At room temperature, hydrogen gas was introduced at a flow rate of 100mL / min. After the air was exhausted, the reaction tube was directly placed in a heating furnace at 600°C, and the catalyst was reduced for 40 minutes. Finally, the reaction tube was directly removed from the heating furnace without turning off the reducing gas. Take it out and place it at room temperature.
[0020] The evaluation of catalyst naphthalene hydrogenation reaction activity is the same as in Example 1, and the results are shown in Table 1.
Embodiment 3
[0022] Weigh 18.5g of nickel acetate and add it into 50g of water, fully stir and dissolve with a magnetic stirrer at 40°C, then add 15g of γ-Al 2 o 3 Carrier, continue to stir, pour out the clear liquid after 5 hours, dry at 150°C for 8 hours, sieve the catalyst into 40-60 mesh particles, and put them into a stainless steel reaction tube with an inner diameter of 12 mm. At room temperature, hydrogen gas was introduced at a flow rate of 57mL / min. After the air was exhausted, the reaction tube was directly placed in a heating furnace at 700°C, and the catalyst was reduced for 10 minutes. Finally, the reaction tube was directly removed from the heating furnace without turning off the reducing gas. Take it out and place it at room temperature.
[0023] The evaluation of catalyst naphthalene hydrogenation reaction activity is the same as in Example 1, and the results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com