Novel medical use of cucurbitacin
A technology of cucurbitacin and medicine, applied in the field of medicine, can solve the problem of reducing serum CA125 in patients with ovarian cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
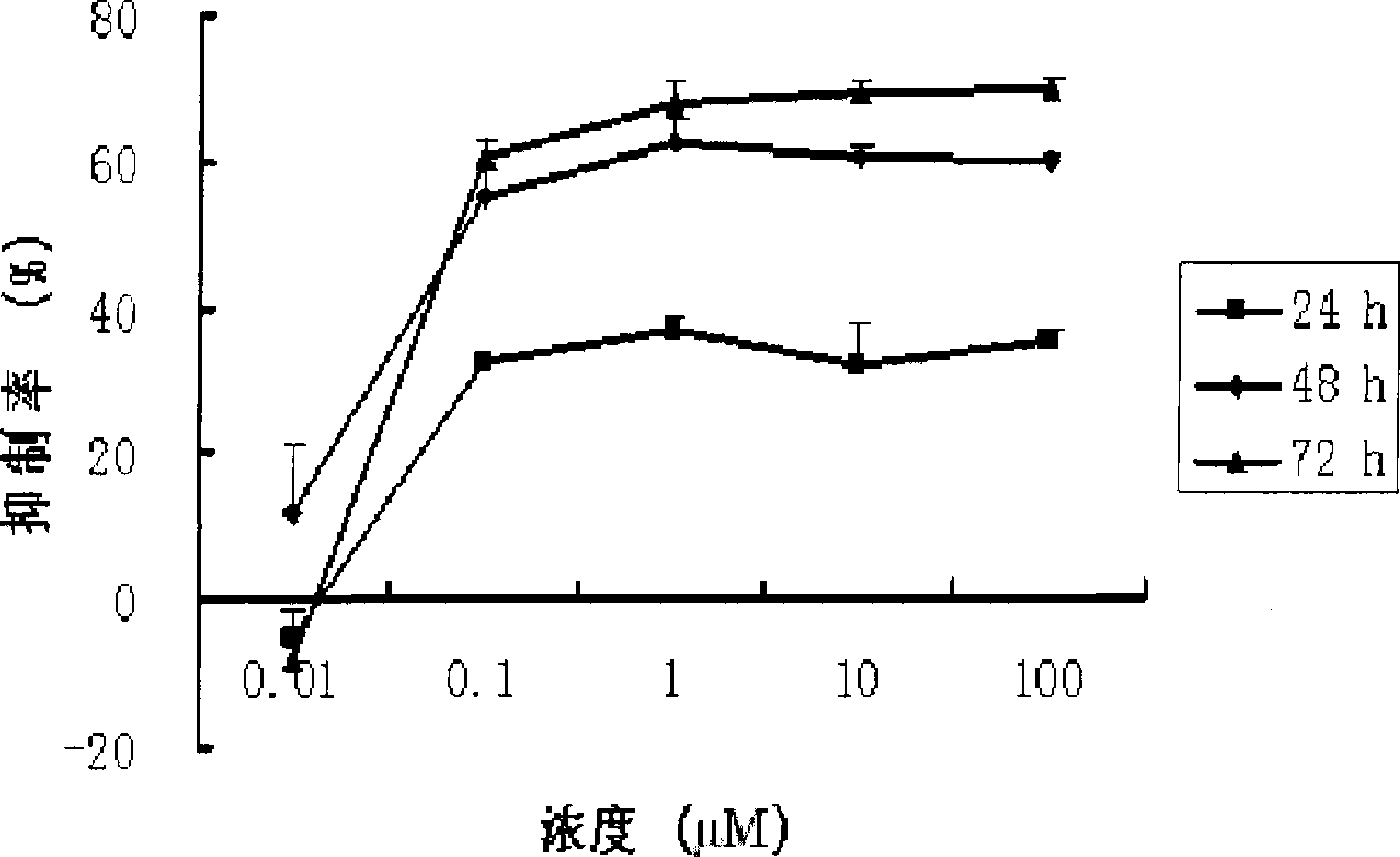
[0022] Cucurbitacin B (CuB, the purity determined by HPLC area normalization method is greater than 99%) in vitro anti-human ovarian cancer cells
[0023] 1. In vitro test (MTT)
[0024] method:
[0025] 1. Human ovarian cancer cell line SK-OV-3 was cultured with RPMI1640 medium containing 10% fetal bovine serum at 37°C and 5% CO 2 In the incubator, subculture once every 3-4 days. Cells in the logarithmic growth phase were selected for the experiment.
[0026] 2. Digest the adherent cells with 0.25% trypsin (containing EDTA 1mM / L) and suspend them in the culture medium to adjust the cell concentration to 2.5×10 4 / mL, inoculate 100 μL per well on a 96-well plate, with 3 replicate wells in each well. Only culture solution was added to blank control wells.
[0027] 3. After culturing for 18-24 hours and waiting for the cells to adhere to the wall, add different concentrations of drugs (0.5 μL) into groups, and the other group is the control group (without adding drugs).
...
Embodiment 2
[0034] Pharmacodynamic study of cucurbitacin B (CuB, the purity determined by HPLC area normalization method is greater than 98%) against ovarian cancer cell line SK-OV-3 in nude mice
[0035] method:
[0036] 18-20 grams of nude mice (BALB / C, 5-6 weeks, female, purchased from the Institute of Experimental Animals, Chinese Academy of Medical Sciences, Chinese Union Medical University) 40, inoculated cells in the right armpit 5 × 10 6 / only, when the tumor grows to be larger than 60mm 3 At the same time, they were randomly divided into 4 groups, which were negative control group (0.9% sodium chloride, 0.2ml / day, intravenous injection), CuB low-dose group (50μg / kg ·day, intravenous injection), middle-dose group ( 100μg / kg·day, intravenous injection), high-dose group (200μg / kg·day, intravenous injection), continuous administration for 14 days. The animals were sacrificed within 24 hours after the last administration, and the tumors were weighed. Calculate the tumor inhibition...
Embodiment 3
[0040] Pharmacodynamic study of dihydrocucurbitacin B (purity determined by HPLC area normalization method greater than 95%) against ovarian cancer cell line SK-OV-3 in nude mice
[0041] Refer to the method of "Example 2"
[0042] 18 nude mice of 18-20 grams, inoculated with 5×10 cells in the right armpit 6 / only, when the tumor grows to be larger than 50mm 3 At the same time, they were randomly divided into 3 groups, which were negative control group (distilled water, intragastric administration, 0.2ml / day), dihydrocucurbitacin B group (gastric administration, 300μg / kg·day), dihydrocucurbitacin group Group B (gastric administration, 400 μg / kg·day), continuous administration for 14 days. The animals were sacrificed within 24 hours after the last administration, and the tumors were weighed to calculate the tumor inhibition rate. Results The tumor inhibition rates of the dihydrocucurbitacin B group were 39.2% and 51.6% respectively; compared with the normal control group, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com