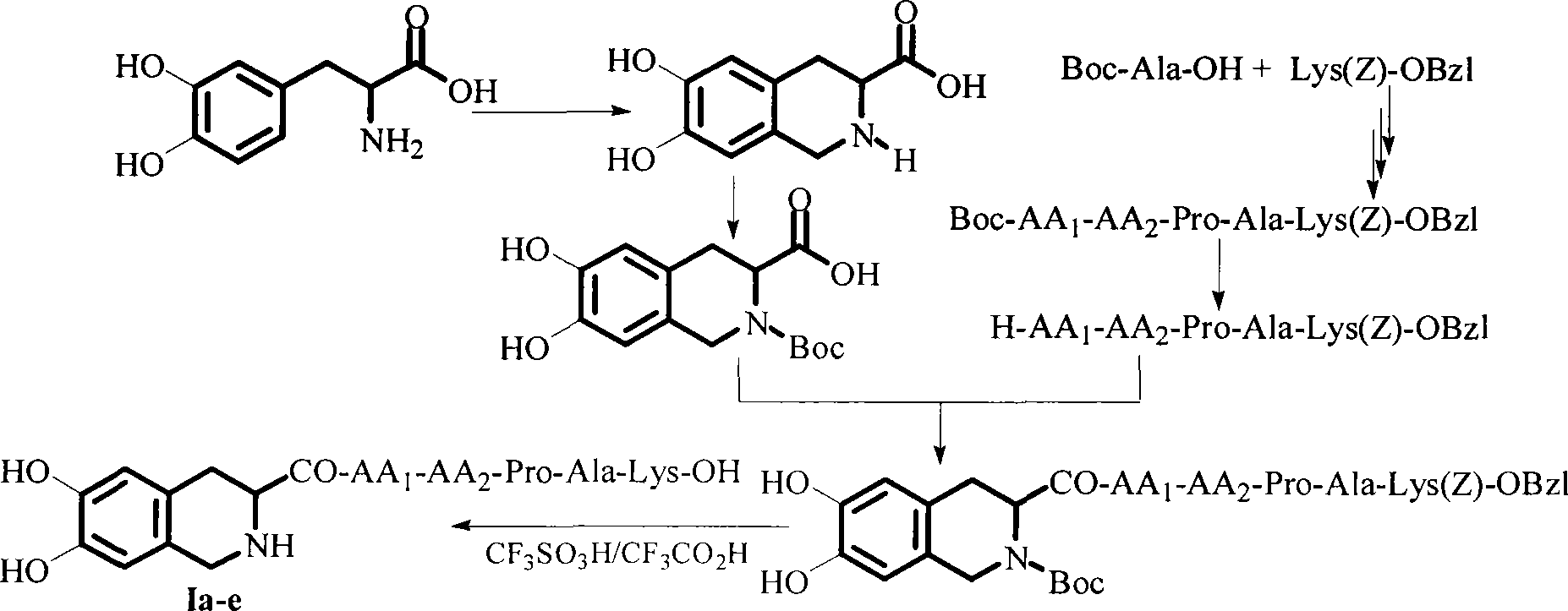
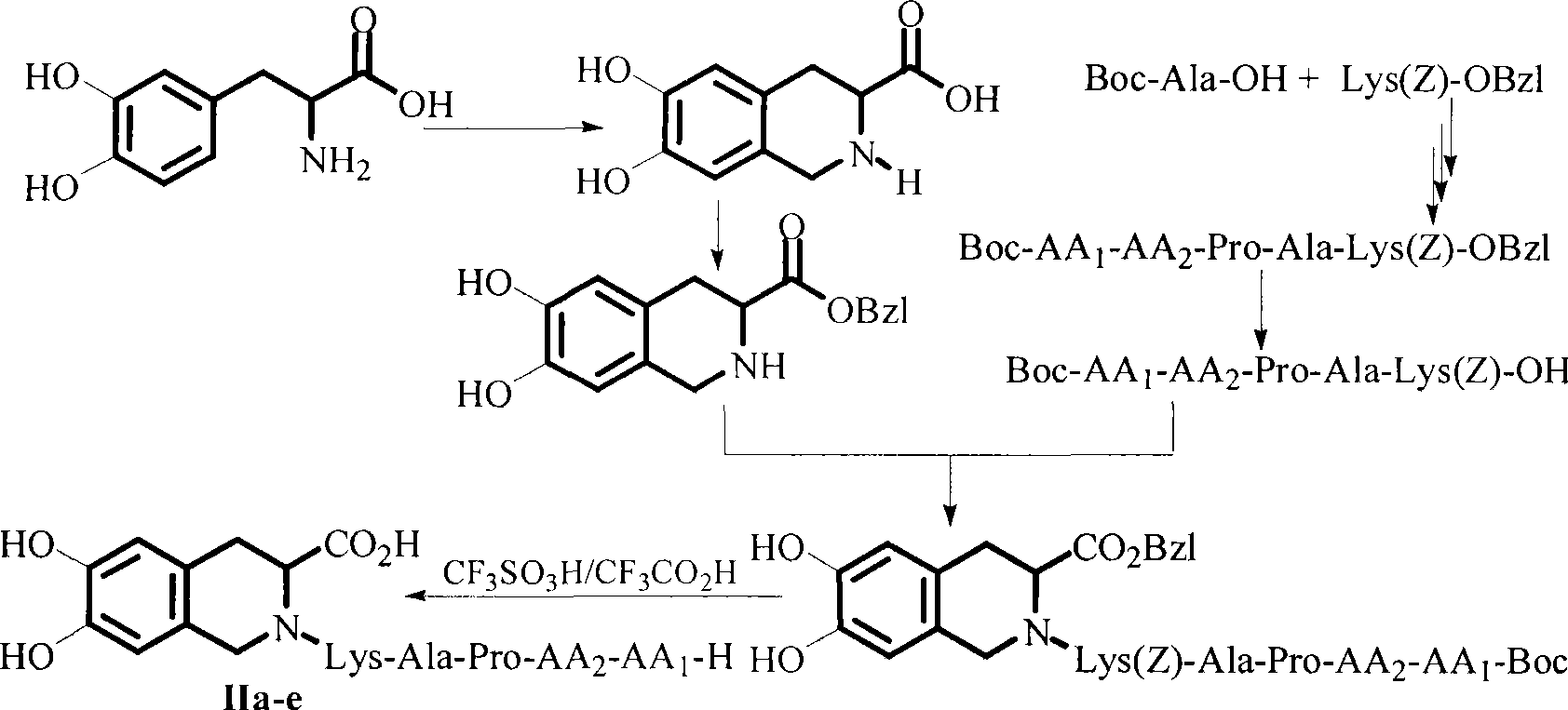
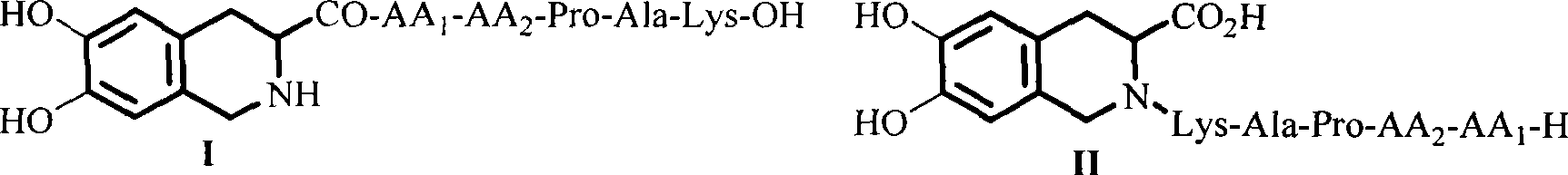Compound with thrombus dissolving activity, as well as preparation and use thereof
A compound, technology of suppository activity, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0042] Example 2 Boc-Arg (NO 2 Preparation of )-Pro-Ala-Lys(Z)-OBzl
[0043] 1) Preparation of HCl Pro-Ala-Lys(Z)-OBzl
[0044] A mixture of 1.596g (2.5mmol) Boc-Pro-Ala-Lys(Z)-OBzl and 15ml hydrogen chloride-ethyl acetate solution (4N) was stirred at room temperature for 3 hours, TLC (developing agent chloroform:methanol=10:1) showed Boc-Pro-Ala-Lys(Z)-OBzl disappears. Drain the reaction solution with a water pump, add ethyl acetate, and drain the reaction solution with a water pump, repeat 5 times. The residue was soaked and washed with anhydrous ether, and the reaction solution was drained again with a water pump, and this was repeated 5 times. The obtained title compound was directly used in the next reaction.
[0045] 2) Preparation of Boc-Arg(NO 2 )-Pro-Ala-Lys(Z)-OBzl
[0046] 798mg (2.5mmol) Boc-Arg (NO 2 )-OH was dissolved in 10ml of anhydrous THF, and 10ml of anhydrous THF solution of 338mg (2.5mmol) HOBt and 619mg (3mmol) DCC was added under ice cooling. The...
Embodiment 3
[0050] Example 3 Boc-Ala-Arg (NO 2 Preparation of )-Pro-Ala-Lys(Z)-OBzl
[0051] 1) Preparation of HCl Arg (NO 2 )-Pro-Ala-Lys(Z)-OBzl
[0052] 2.099g (2.5mmol) Boc-Arg (NO 2 )-Pro-Ala-Lys(Z)-OBzl and the mixture of 15ml hydrogen chloride-ethyl acetate solution (4N) were stirred at room temperature for 3 hours, TLC (developing agent chloroform:methanol=10:1) showed that Boc-Arg(NO 2 )-Pro-Ala-Lys(Z)-OBzl disappeared. Drain the reaction solution with a water pump, add anhydrous ether, and drain the reaction solution with a water pump again, repeating 5 times. The residue was soaked and washed with anhydrous ether, rubbed with a plastic shovel, and poured out of anhydrous ether, repeated 5 times. The obtained title compound was directly used in the next reaction.
[0053] 2) Preparation of Boc-Ala-Arg (NO 2 )-Pro-Ala-Lys(Z)-OBzl
[0054] Dissolve 473mg (2.5mmol) Boc-Ala-OH in 10ml of anhydrous THF, and add 10ml of anhydrous THF solution of 338mg (2.5mmol) HOBt and 619mg ...
Embodiment 4
[0058] Example 4 Boc-Gly-Arg (NO 2 Preparation of )-Pro-Ala-Lys(Z)-OBzl
[0059] 1) Preparation of Boc-Gly-Arg (NO 2 )-Pro-Ala-Lys(Z)-OBzl
[0060] Dissolve 438mg (2.5mmol) of Boc-Gly-OH in 10ml of anhydrous THF, and add 10ml of anhydrous THF solution of 338mg (2.5mmol) of HOBt and 619mg (3mmol) of DCC under ice-cooling. The reaction mixture was stirred in an ice bath for 20 minutes to obtain the corresponding active ester solution for use.
[0061] 2.025g (2.3mmol) HCl Arg (NO 2 )-Pro-Ala-Lys(Z)-OBzl and 232mg (2.3mmol) N-methylmorpholine were first miscible with 6ml of anhydrous THF, and then with the active ester solution to be used above. The resulting reaction mixture was reacted at room temperature for 24 hours. TLC (developing agent chloroform:methanol=10:1) shows that HCl Arg (NO 2 )-Pro-Ala-Lys(Z)-OBzl disappeared. The reaction mixture was concentrated to dryness under reduced pressure, the residue was dissolved in ethyl acetate, and the insoluble matter was fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com