Double-functional molecule for dissolving thrombus and inhibiting blood platelet aggregate and use thereof
A platelet aggregation and bifunctional molecule technology, applied in the biological field, can solve the problems of difficult chemical cross-linking operation, increased cost, easy loss of activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
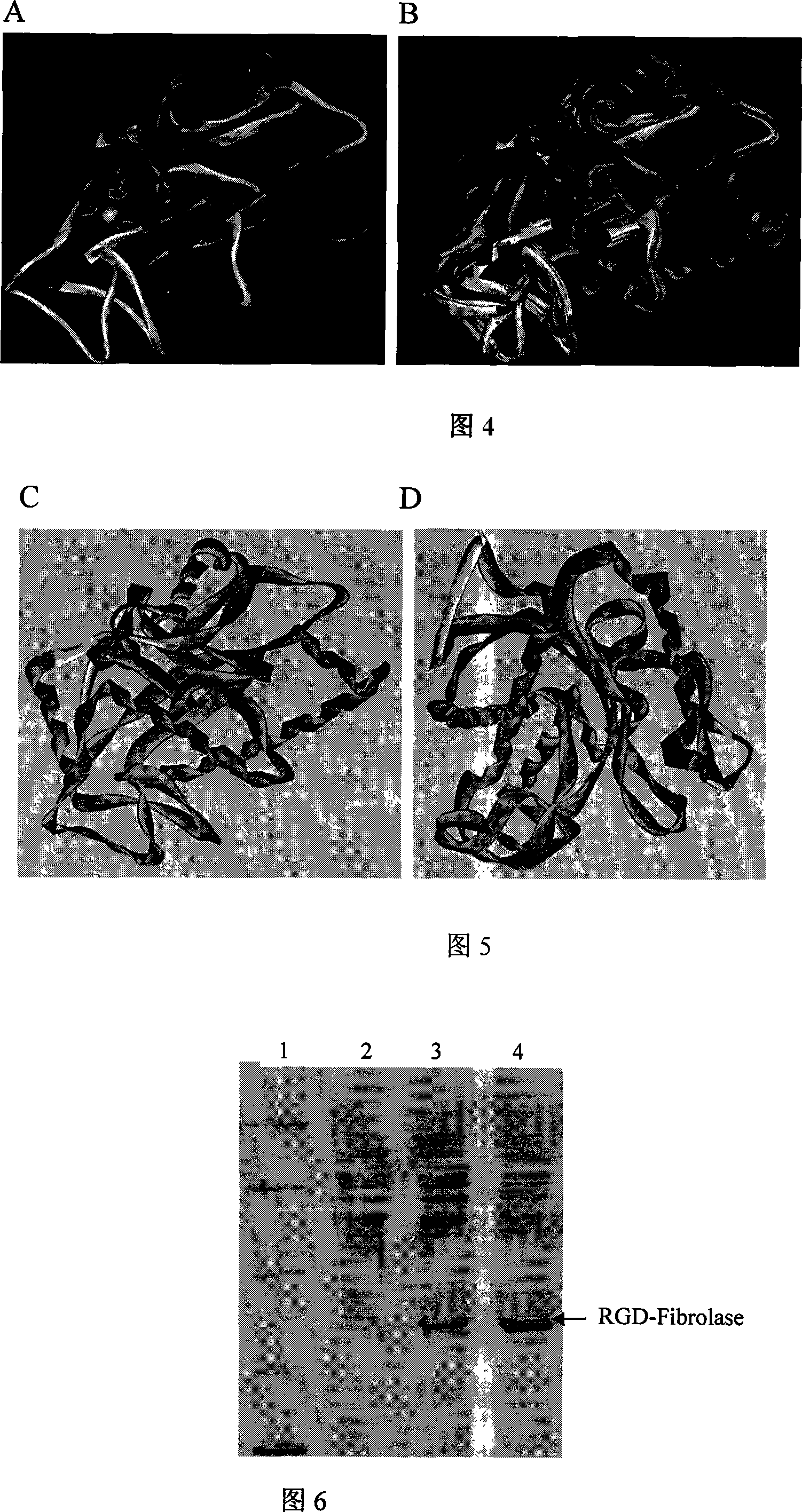
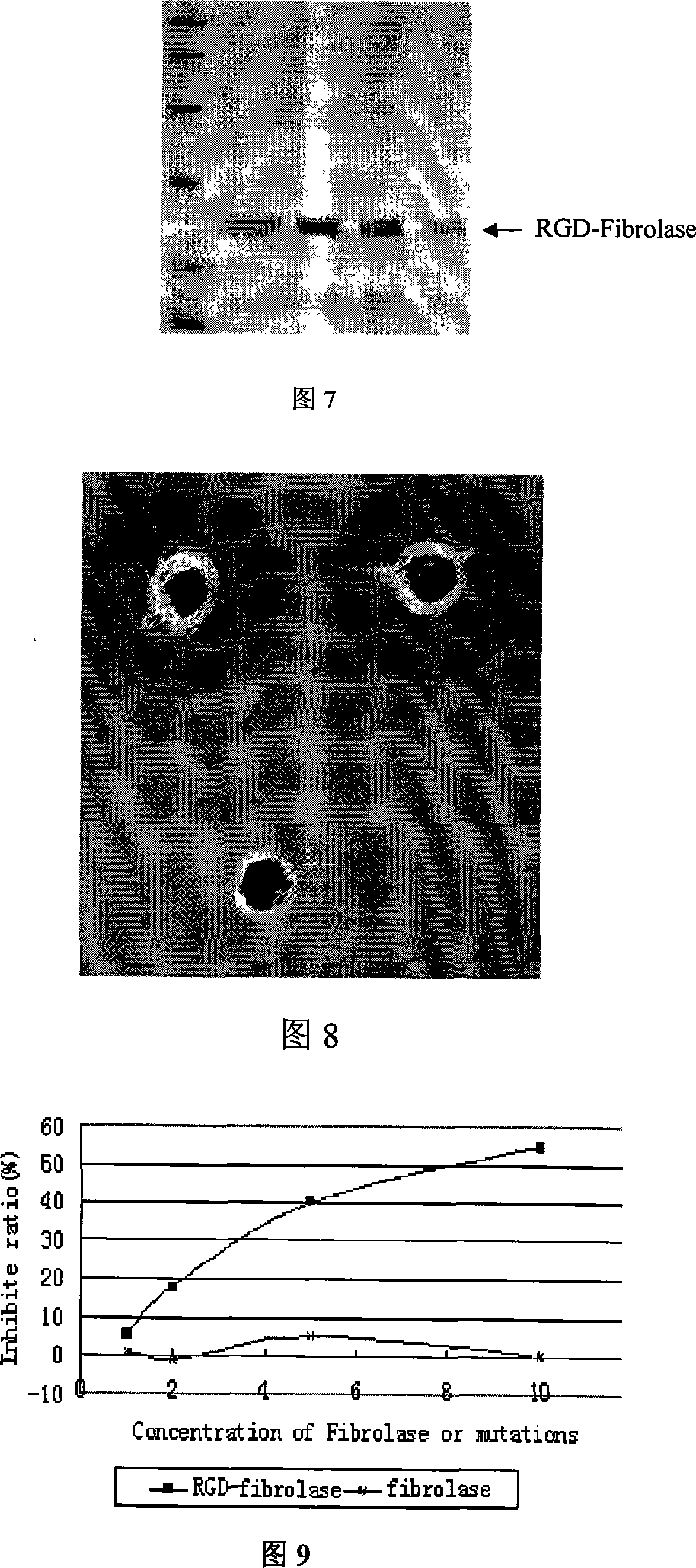
[0036] In the RGD bifunctional molecule for thrombolysis / inhibition of platelet aggregation of the present invention, the RGD coding sequence is fused to the wild-type Fibrolase gene when synthesizing the RGD-Fibrolase gene.
[0037] According to the structural characteristics and mode of action of Fibrolase, a dual-functional RGD-Fibrolase molecular structure with the ability to dissolve thrombus and inhibit platelet aggregation was designed, and produced by genetic engineering. new properties, and the preparation process is simple and safe.
[0038] From the three-dimensional structure of Fibrolase, amino acids 69-74 are located outside the molecular structure, presenting a prominent Loop shape, away from the known active center. The introduction of the RGD sequence at this position can not only make the RGD better play the anticoagulant function, but also have less impact on the activity of Fibrolase, and maintain the fibrolase fibrolytic activity well.
[0039] The RGD bi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com