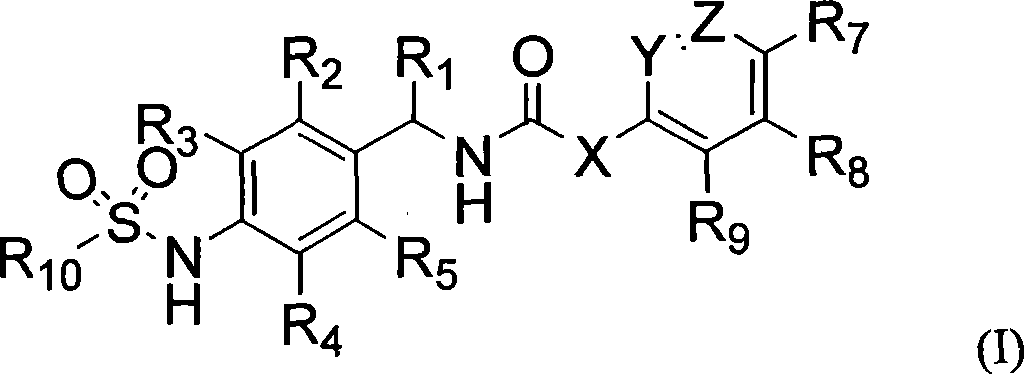
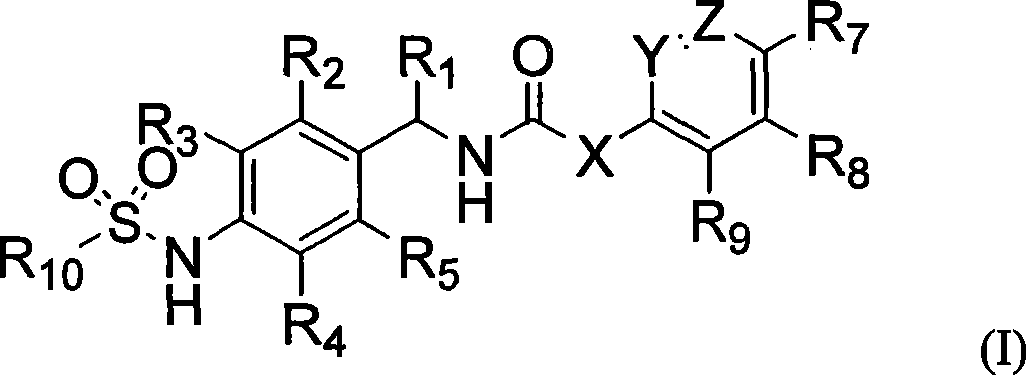
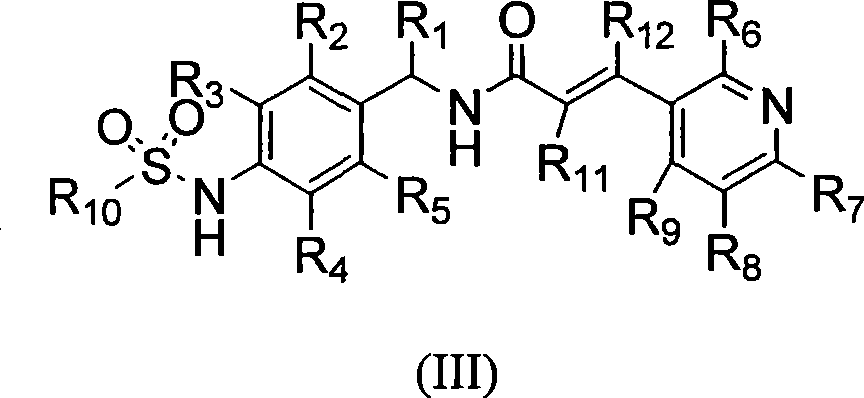
Novel compounds, isomer thereof, or pharmaceutically acceptable salts thereof as vanilloid receptor antagonist
A compound and pharmaceutical technology, applied in the field of pharmaceutical compositions containing these compounds, can solve problems such as nerve function damage and nerve cell death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0912] Example 1: 3-(6-tert-butyl-pyridin-3-yl)-N-(3-fluoro-4-methanesulfonylamino-benzyl)-acrylamide
[0913]
[0914] Step 1: Synthesis of 3-(6-tert-butyl-pyridin-3-yl)-acrylic acid
[0915] To a solution of 6-tert-butyl-pyridine-3-carbaldehyde (1.34 g, 8.75 mmol) in toluene prepared according to a known method was added methyl (triphenylphosphino)acetate (2.93 g) to give The mixture was heated at 90°C for 3 hours. The reaction mixture was diluted with EtOAc, then washed with water and brine. Several layers were dried over anhydrous MgSO4 and concentrated under reduced pressure. The resulting residue was purified by column chromatography (Hex / EtOAc=4 / 1) to give the ester product (1.56 g, 81%). The resulting ester was dissolved in 1,4-dioxane, treated with water and KOH, stirred, then heated at reflux for 18 hours. The reaction mixture was cooled to room temperature, diluted with water, and washed with ether. The aqueous phase was acidified with 1N HCl, followed by CH...
Embodiment 2
[0921] Example 2: 3-(6-tert-butyl-pyridin-3-yl)-N-(3-chloro-4-methanesulfonylamino-benzyl)-acrylamide
[0922]
[0923] N-(4-Aminomethyl-2-chloro-phenyl)-methanesulfonamide, HCl salt (100mg, 0.35mmol) and 3-(6-tert-butyl-pyridin-3-yl)-acrylic acid (70mg) The reaction afforded 3-(6-tert-butyl-pyridin-3-yl)-N-(3-chloro-4-methanesulfonylamino-benzyl after column chromatography purification (Hex / EtOAc=1 / 2) )-acrylamide (110 mg, 74%).
[0924] 1 H NMR (300MHz, CDCl 3 ): δ 8.66 (d, 1H, J = 2.1Hz), 7.74 (dd, 1H, J = 2.1 and 8.1Hz), 7.64 (d, 1H, J = 15.6Hz), 7.57 (d, 1H, J = 8.7 Hz), 7.41(d, 1H, J=2.1Hz), 7.36(d, 1H, J=8.1Hz), 7.24(dd, 1H, J=2.1 and 8.7Hz), 6.82(s, 1H), 6.48( d, 1H, J=15.6Hz), 6.42(t, 1H), 4.53(d, 2H, J=6.0Hz), 3.00(s, 3H), 1.37(s, 9H)
[0925] ESI[M+H] + : 422.2.
Embodiment 3
[0926] Example 3: 3-(6-tert-butyl-pyridin-3-yl)-N-(3-ethynyl-5-fluoro-4-methanesulfonylamino-benzyl)-acrylamide
[0927]
[0928] N-(4-Aminomethyl-2-ethynyl-6-fluoro-phenyl)-methanesulfonamide, HCl salt (70mg, 0.25mmol) and 3-(6-tert-butyl-pyridin-3-yl) - reaction of acrylic acid (52 mg) to give 3-(6-tert-butyl-pyridin-3-yl)-N-(3-ethynyl-5-fluoro after purification by column chromatography (Hex / EtOAc=1 / 2) - 4-Methanesulfonylamino-benzyl)-acrylamide (63 mg, 64%).
[0929] 1 H NMR (300MHz CDCl 3 ): δ 8.71 (d, 1H, J = 2.4Hz), 7.76 (dd, 1H, J = 2.4 and 8.4Hz), 7.63 (d, 1H, J = 16.0Hz), 7.39 (d, 1H, J = 8.4 Hz), 7.28(s, 1H), 7.16(dd, 1H, J=2.1 and 11.0Hz), 6.64(s, 1H), 6.52(d, 1H, J=16.0Hz), 6.47(t, 1H), 4.51(d, 2H, J=6.0Hz), 3.45(s, 1H), 3.24(s, 3H), 1.38(s, 9H)
[0930] ESI[M+H] + : 430.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com