Ophthalmic percutaneous absorption type preparation
An absorption-type, ophthalmic technology, applied in medical preparations containing active ingredients, allergic diseases, drug combinations, etc., can solve the problems of adding ophthalmic drugs, not described, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
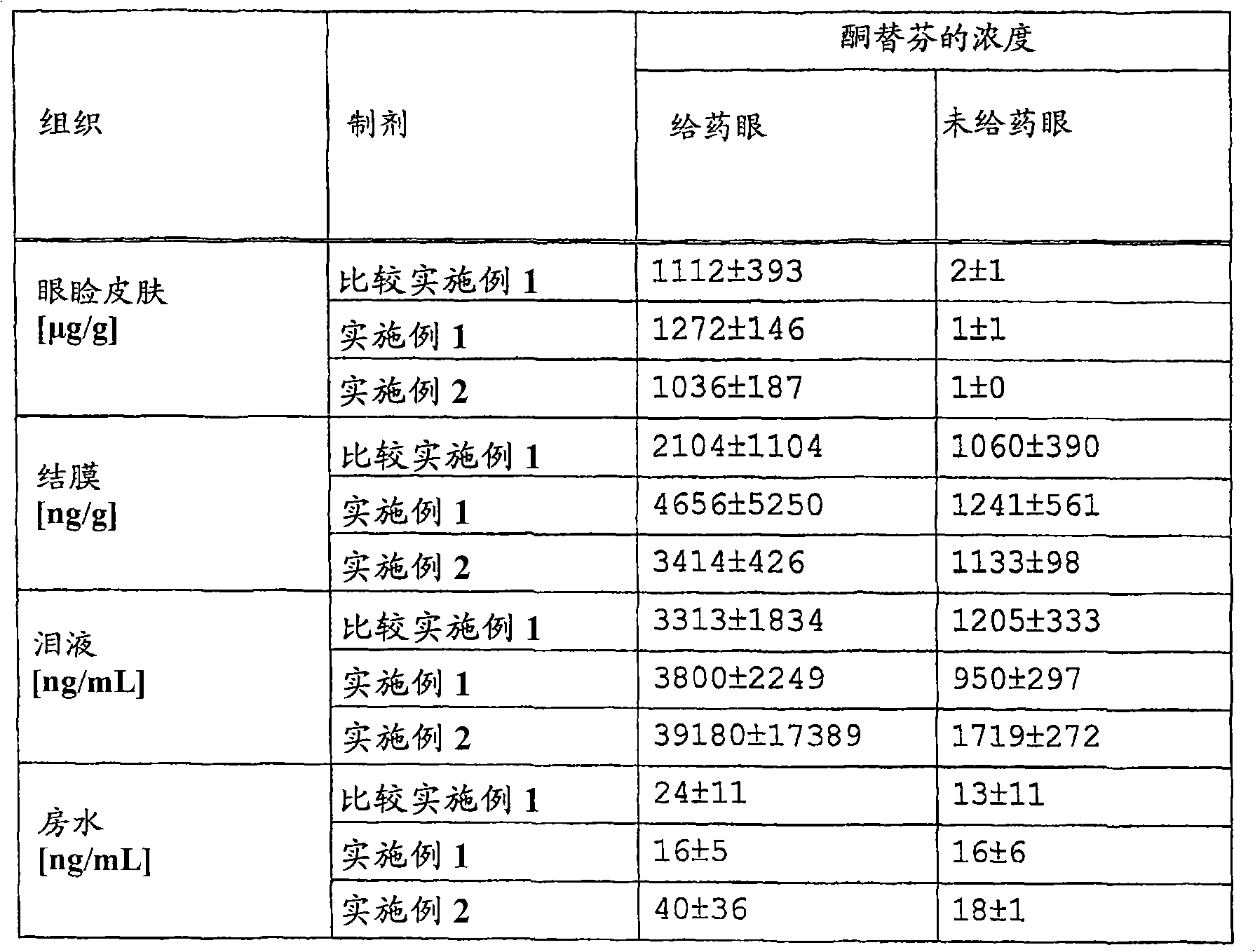
[0092] Although the present invention can be explained in more detail with reference to the following Experimental Examples and Examples, they should not be construed as limiting the present invention.
[0093] Test Method and Results
[0094]
[0095] Ketotifen fumarate (Sigma Co., Ltd.), phenylephrine hydrochloride (for biochemistry, Wako Pure Chemical Co., Ltd.), hydroxypropyl methylcellulose (Metolose 60SH-4000, Shin-Etsu Chemical Co., Ltd.), phosphoric acid Sodium dihydrogen dihydrate (first-class product, Wako Pure Chemical Co., Ltd.) and sodium hydroxide (Japanese Pharmacopoeia, Nacalai Tesque).
[0096]
Embodiment 1
[0098] Example 1: Gel formulation containing 20% ketotifen fumarate and 2% phenylephrine hydrochloride
Embodiment 2
[0099] Example 2: Gel formulation containing 20% ketotifen fumarate and 4% phenylephrine hydrochloride
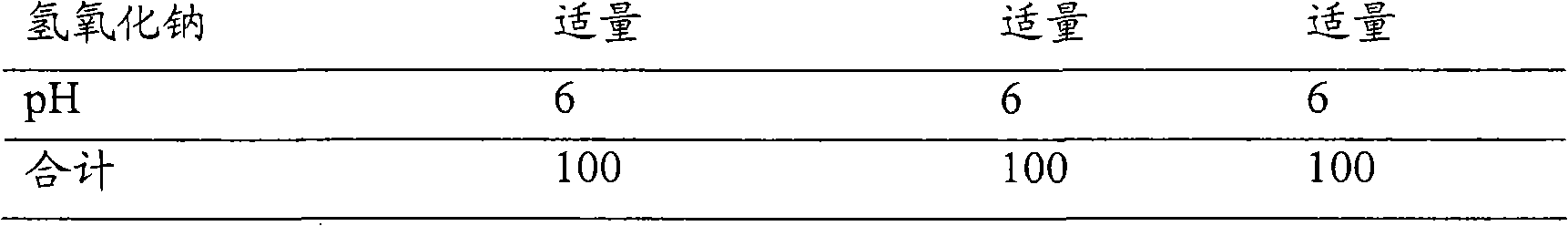
[0100] These formulations were prepared according to the formulations in Table 1 and the formulation methods mentioned below.
[0101] Table 1
[0102]
[0103]
[0104] (Unit: w / w%)
[0105]
[0106] Comparative example 1 preparation
[0107] Sodium dihydrogenphosphate dihydrate was added to purified water, and the mixture was stirred until completely dissolved. Heat the solution to about 70°C in a water bath, add hydroxypropyl methylcellulose in small amounts and stir to dissolve. After standing at room temperature for 10 minutes, 1N aqueous sodium hydroxide solution was added. The pH of the mixture was adjusted to 6 to obtain a gel matrix. Ketotifen fumarate and gel matrix were measured on a glass petri dish, and stirred well with a spatel stirrer to obtain a formulation of Comparative Example 1 (gel formulation containing 20% ketotifen fumarate).
[01...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com