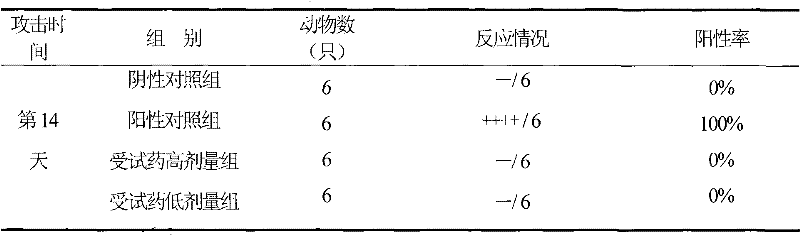
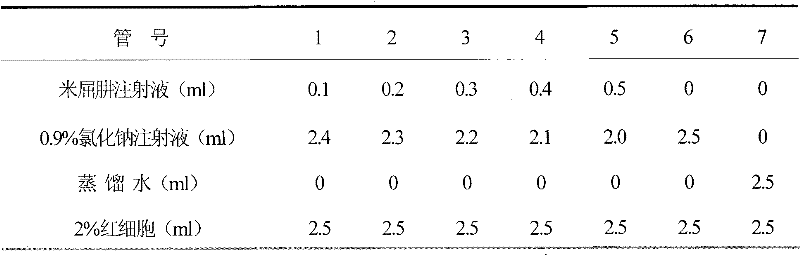
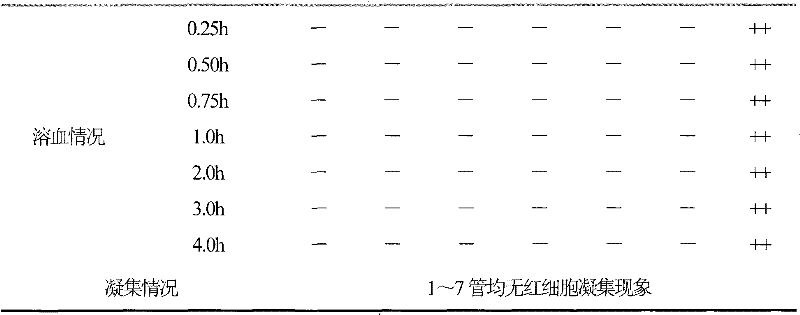
Formulation of mildronate injection and preparation method
A technology of meldonium and injection, which is applied in the field of pharmaceutical preparations and can solve problems such as the inability to adopt terminal sterilization technology to sterilize
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Preparation of Meldonium Injection
[0035] Weigh meldonium [3-(2,2,2-trimethylhydrazine) propionate dihydrate] 100g into the mixing tank, add 750ml of water for injection into the mixing tank, stir to make the main ingredient dissolve completely, Weigh 1.97g of disodium hydrogen phosphate into the mixing tank, stir and mix until completely dissolved, then weigh 0.23g of potassium dihydrogen phosphate, add it into the mixing tank, stir and mix until completely dissolved, add water for injection to 1000ml, mix well, Add 0.05% (W / V) activated carbon to the solution, stir for 15 minutes, pressurize the liquid to pass through 1 μm and 0.45 μm titanium rod filters wrapped with neutral filter paper, filter the activated carbon into the dilute tank, and take samples Check the pH value of the solution. If the pH value is within the acceptable range, no adjustment is required. Take the qualified filtrate and use a 0.45μm filter membrane for coarse filtration, and then use a 0.22...
Embodiment 2
[0037] Preparation of Meldonium Injection
[0038] Take by weighing Meldonium [3-(2,2,2-trimethylhydrazine) propionate dihydrate] 105g to the mixing tank, add 750ml of water for injection to the mixing tank, stir to make the main ingredient dissolve completely, Weigh 5.6g of dipotassium hydrogen phosphate and add it to the mixing tank, stir and mix until completely dissolved, then weigh 0.4g of potassium dihydrogen phosphate and add it to the mixing tank, stir and mix until completely dissolved, add water for injection to 1000ml, mix well, Add 0.05% (W / V) activated carbon to the solution, stir for 15 minutes, pressurize the liquid to pass through a 1 μm and 0.45 μm titanium rod filter wrapped with neutral filter paper, filter the activated carbon into the diluted tank, and take a sample Check the pH value of the solution. If the pH value is within the acceptable range, no adjustment is required. Take the qualified filtrate and use a 0.45μm filter membrane for coarse filtration...
Embodiment 3
[0040] Preparation of Meldonium Injection
[0041] Weigh meldonium [3-(2,2,2-trimethylhydrazine) propionate dihydrate] 90g into the mixing tank, add 750ml of water for injection into the mixing tank, stir to make the main ingredient dissolve completely, Weigh 6.8g of potassium dihydrogen phosphate and add it to the mixing tank, stir and mix until it is completely dissolved, then weigh 1.7g of sodium hydroxide and add it to the mixing tank, stir and mix until it is completely dissolved, add water for injection to 1000ml, mix well, and add Add 0.05% (W / V) activated carbon to the solution, stir for 15 minutes, pressurize the liquid to pass through 1 μm and 0.45 μm titanium rod filters wrapped with neutral filter paper, filter the activated carbon into the dilute tank, and take samples for inspection The pH value of the solution, the pH value is within the qualified range, no adjustment is required, the qualified filtrate is firstly filtered with a 0.45 μm filter membrane, and then ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com