Non-integration lentiviral vector system and preparation and application thereof
A lentiviral vector, non-integrating technology, applied in the lentiviral vector system and its preparation and application fields, can solve the problem of no manufacturer providing non-integrating lentiviral vectors, and achieve the effects of long-term gene expression and efficient gene transfer.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] 1 Preparation of carrier
[0069] 1.1 Experimental process description
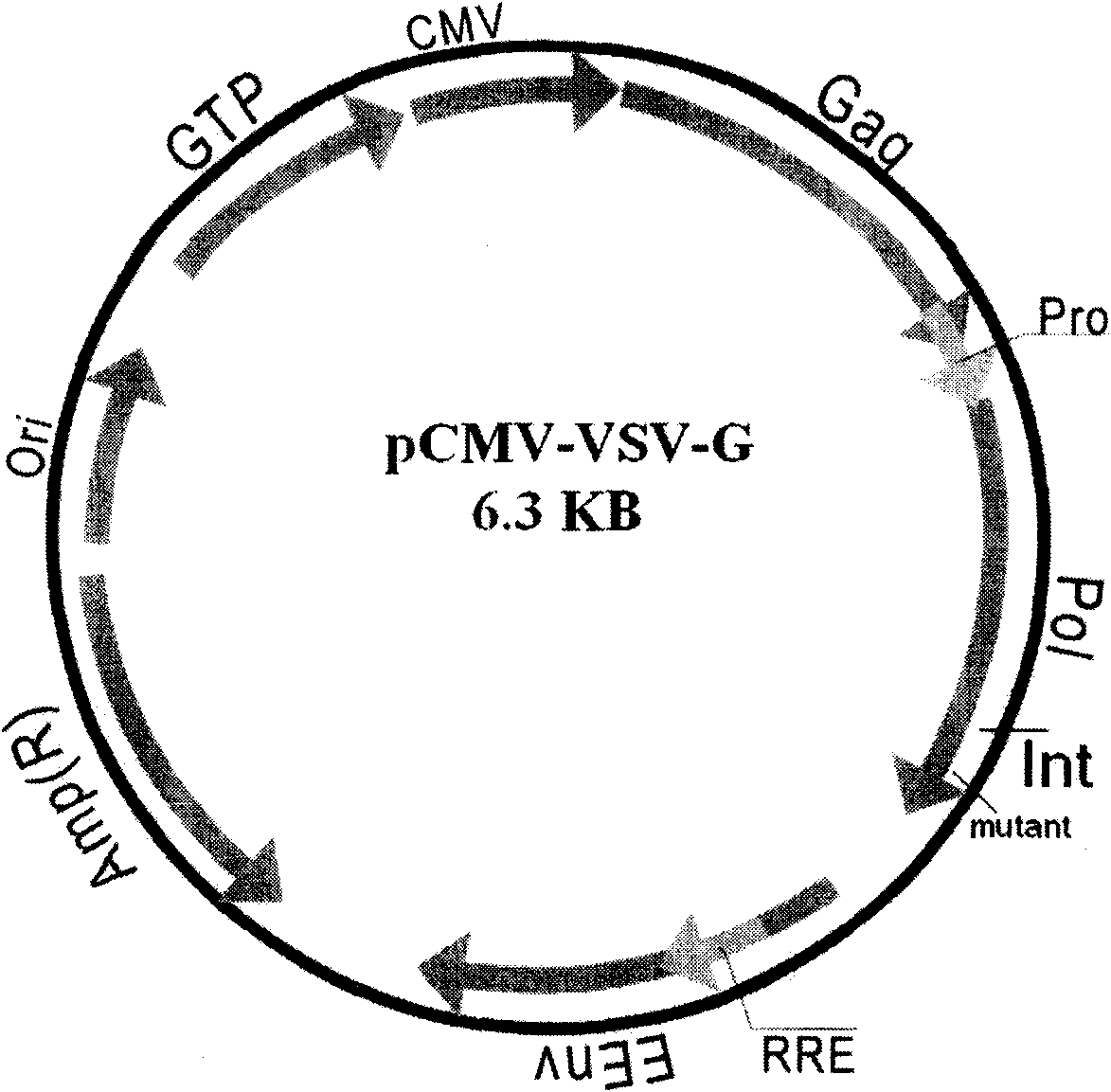
[0070] From the plasmid pCMV-dR8.2dvpr plasmid template containing the HIV integrase gene, the target gene was caught by PCR method, and the target gene and the target vector were digested respectively. Purify the digested product and carry out directional ligation. The product is transformed into bacterial competent cells. The grown clones are first identified by PCR. The upstream and downstream primers are respectively designed on the carrier and the target gene. The positive clones identified by PCR prove the target gene. Already directional ligated into the destination vector. The positive clones identified by PCR were then sequenced, analyzed and compared, and the correct comparison was the successful construction of fusion protein expression plasmid vectors. The constructed fusion protein expression vector was subjected to ultrapure endotoxin-free extraction.
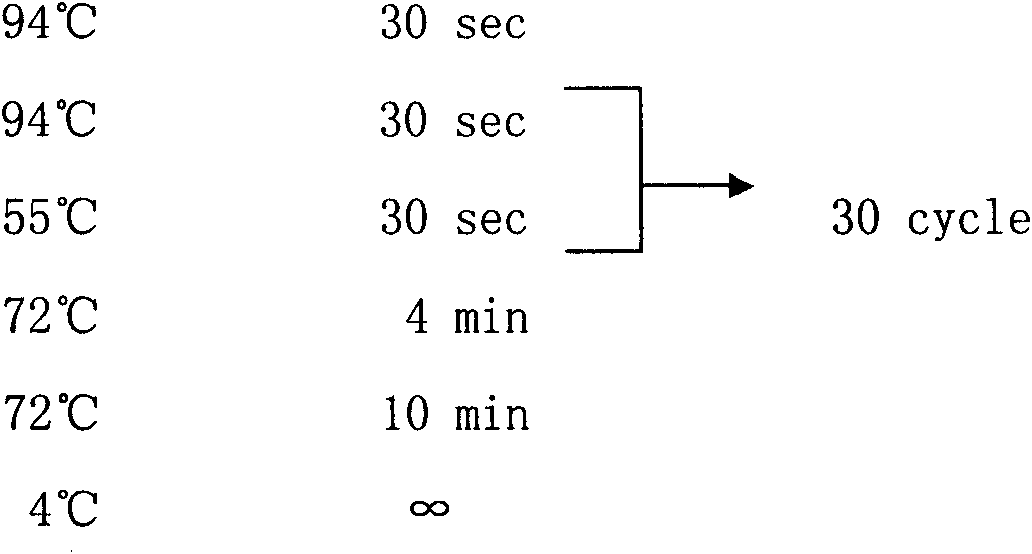
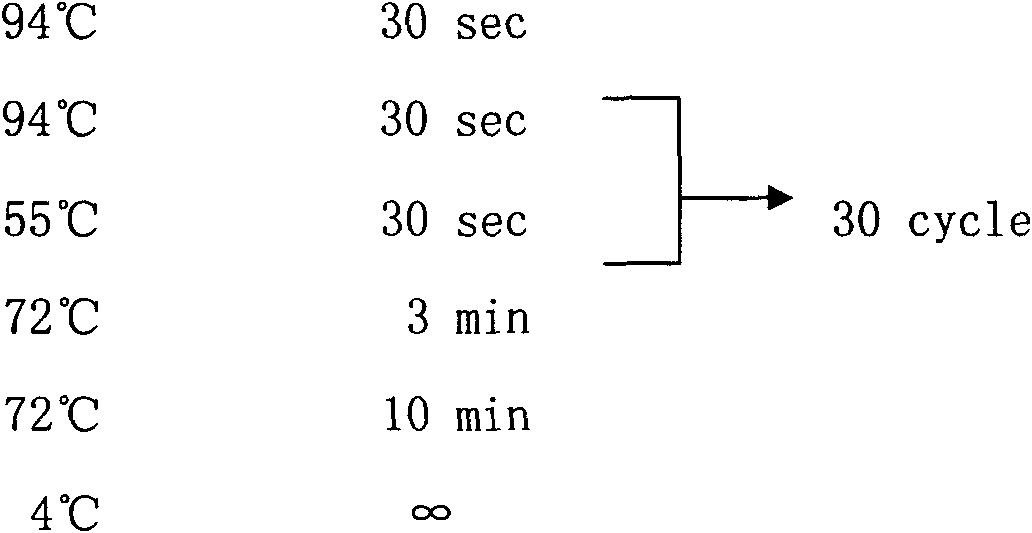
[0071] 1.2 Experimental flow...
Embodiment 2 Embodiment 1
[0256] Example 1, comparison of GFP conventional lentivirus and GFP non-integrated lentivirus:
example 1
[0257] 1. Cloning the GFP gene into the pLVTH vector:
[0258] Steps: Obtain the target gene GFP by PCR method, digest the PCR product and pLVTH vector, and ligate the digested product with DNA ligase to obtain a recombinant plasmid; transform the recombinant plasmid into competent cells, and screen positive clones by PCR For the cell line, further identify whether the inserted sequence in the positive clone is correct by sequencing; for the correct positive clone, expand the culture, extract the plasmid, and obtain the pGC GFP LV recombinant plasmid DNA.
[0259] 2. Obtain GFP non-integrating lentivirus (mutant GFP LV) according to the method of steps 3-5 in Example 1.
[0260] 3. According to the method of steps 3-5 in Example 1, the only difference is that the pCMV-dR8.2dvpr vector is used instead of the pHelper 1.0 vector to obtain a conventional GFP lentivirus (wild type GFP LV).
[0261] 4. Infect 293T cells with the GFP non-integrating lentiviruses prepared in steps 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com