Dual-carbodiimide class and preparation as well as anti-hydrolysis application thereof
A biscarbodiimide and carbodiimide technology, applied in the field of biscarbodiimide compounds and their preparation, can solve problems such as difficult metering units
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
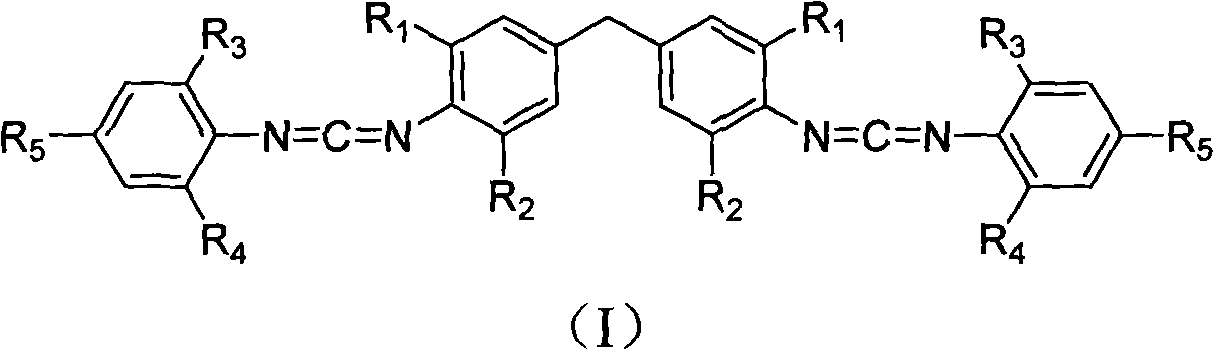
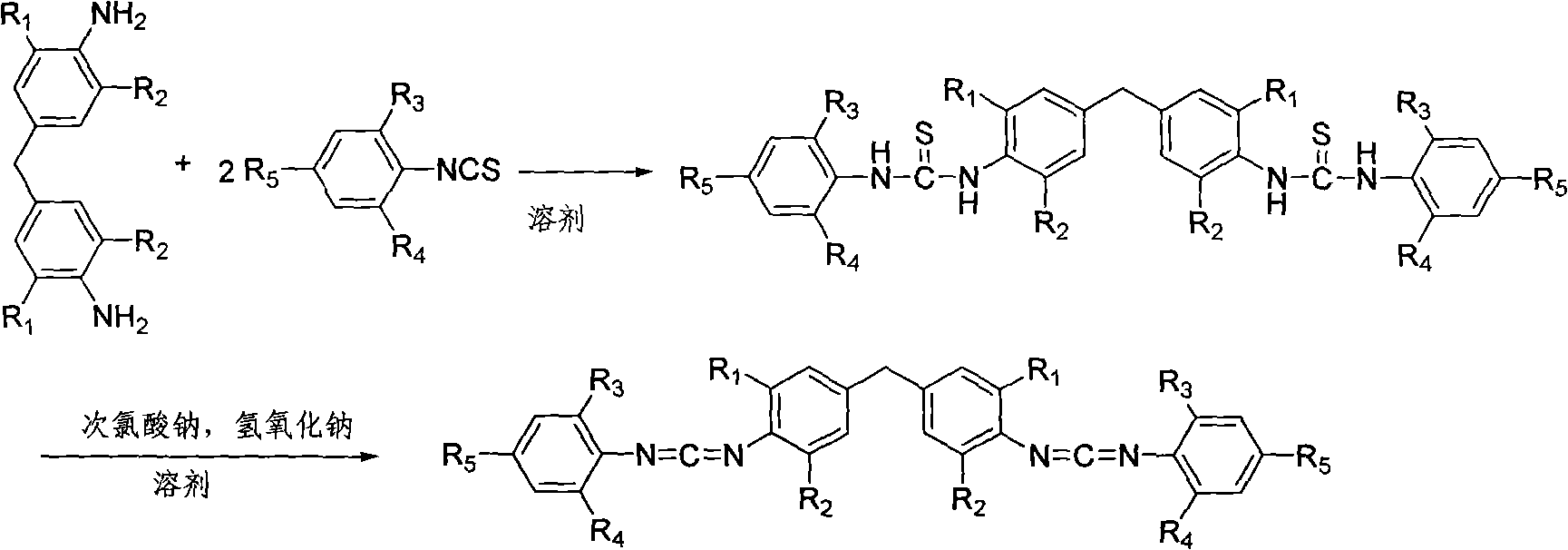
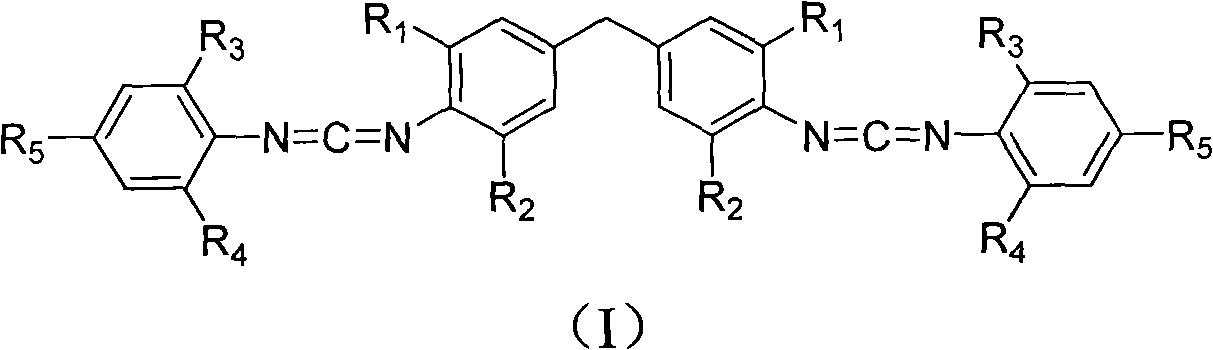
[0024] 3.66 kilograms of 4,4-methylene bis(2,6-diisopropylaniline) and 4.38 kilograms of 2,6-diisopropylphenyl isothiocyanate were added to the reactor, followed by injection of 40 liters Ethylene dichloride was dissolved by mechanical stirring, then the temperature was raised to 80°C for addition reaction, and the heat preservation reaction was completely converted to the dithiourea intermediate in 8 hours; directly added 8 kilograms of 20%wt sodium hydroxide solution to the suspension, and Under stirring, add dropwise 44.7 kilograms of 10%wt sodium hypochlorite aqueous solution in 2 hours, after dripping, continue stirring reaction at room temperature for 12 hours, finish reaction; Suction filtration obtains by-product sulfur; Atmospheric distillation reclaims dichloroethane to obtain the crude product, the crude product is recrystallized with 50 liters of methanol, and after drying, 6.26 kg of white crystal biscarbodiimide compounds (I-1) are obtained, and the high-resolutio...
Embodiment 2
[0026] 3.1 kilograms of 4,4-methylenebis(2,6-diethylaniline) and 4.38 kilograms of 2,6-diisopropylphenyl isothiocyanate were added to the reactor, followed by injection of 45 liters of diethylaniline Ethyl chloride was dissolved by mechanical stirring, and then the temperature was raised to 70°C for addition reaction, and the heat preservation reaction was completely converted into the dithiourea intermediate for 2 hours; 10 kg of 20%wt sodium hydroxide solution was directly added to the suspension, and stirred at room temperature 59.6 kilograms of 10%wt sodium hypochlorite aqueous solution was added dropwise in 1 hour, and after dripping, the stirring reaction at room temperature was continued for 7 hours, and the reaction was terminated; the by-product sulfur was obtained by suction filtration; Obtain crude product after pressure distillation reclaims ethylene dichloride, crude product recrystallizes with 45 liters of methanol, obtains 5.92 kilograms of white crystal biscarbo...
Embodiment 3
[0028] 3.1 kilograms of 4,4-methylene bis(2,6-diethylaniline) and 4.94 kilograms of 2,6-di-tert-butylphenyl isothiocyanate were added to the reactor, followed by injection of 45 liters of diethylaniline Ethyl chloride, mechanically stirred and dissolved, carried out addition reaction at 20°C, and the insulation reaction was completely converted to dithiourea intermediate for 8 hours; directly added 9 kilograms of 20%wt sodium hydroxide solution to the suspension, and stirred at room temperature, Add 58 kilograms of 10% wt sodium hypochlorite solution dropwise within 2 hours, continue to stir the reaction at room temperature for 12 hours after the drop is completed, and end the reaction; suction filtration obtains the by-product sulfur; After recovering dichloroethane, the crude product was obtained. The crude product was recrystallized with 45 liters of methanol, and after drying, 5.9 kilograms of white crystal biscarbodiimide compounds (I-3) were obtained. High-resolution mass...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com