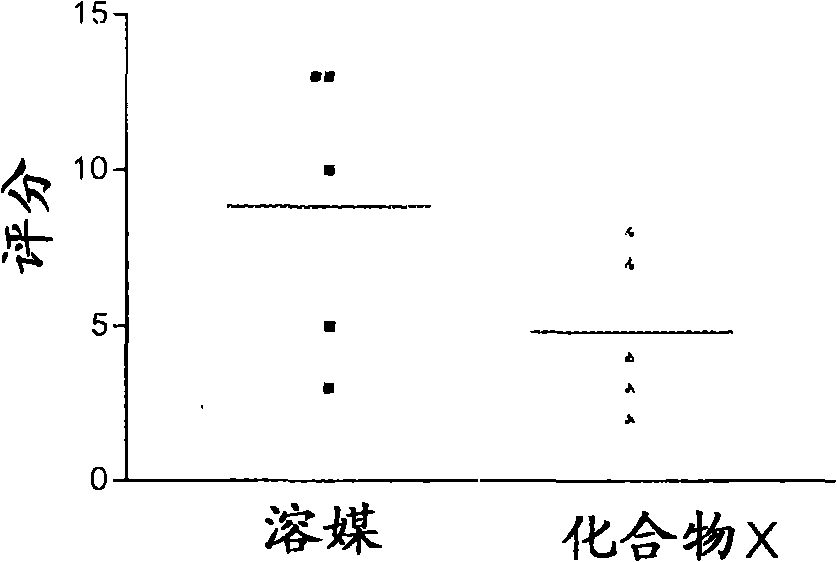
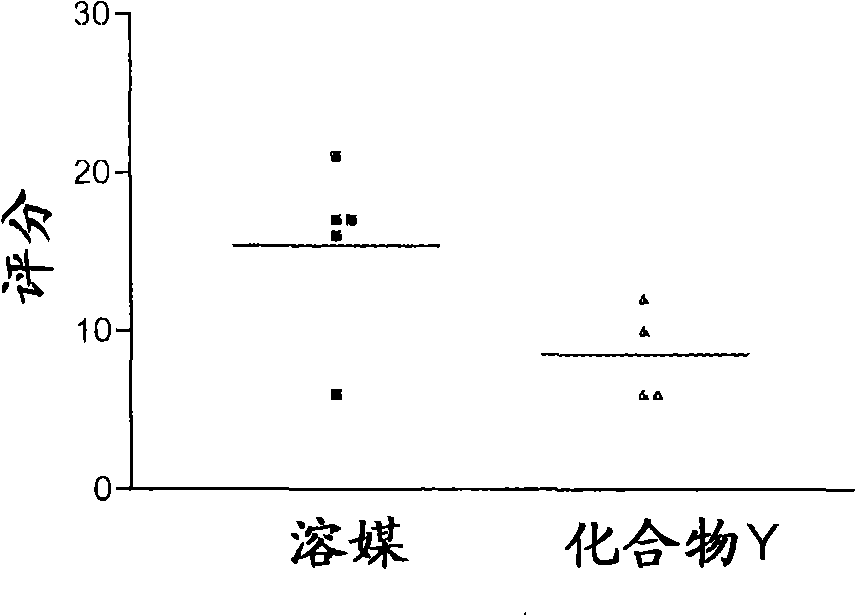
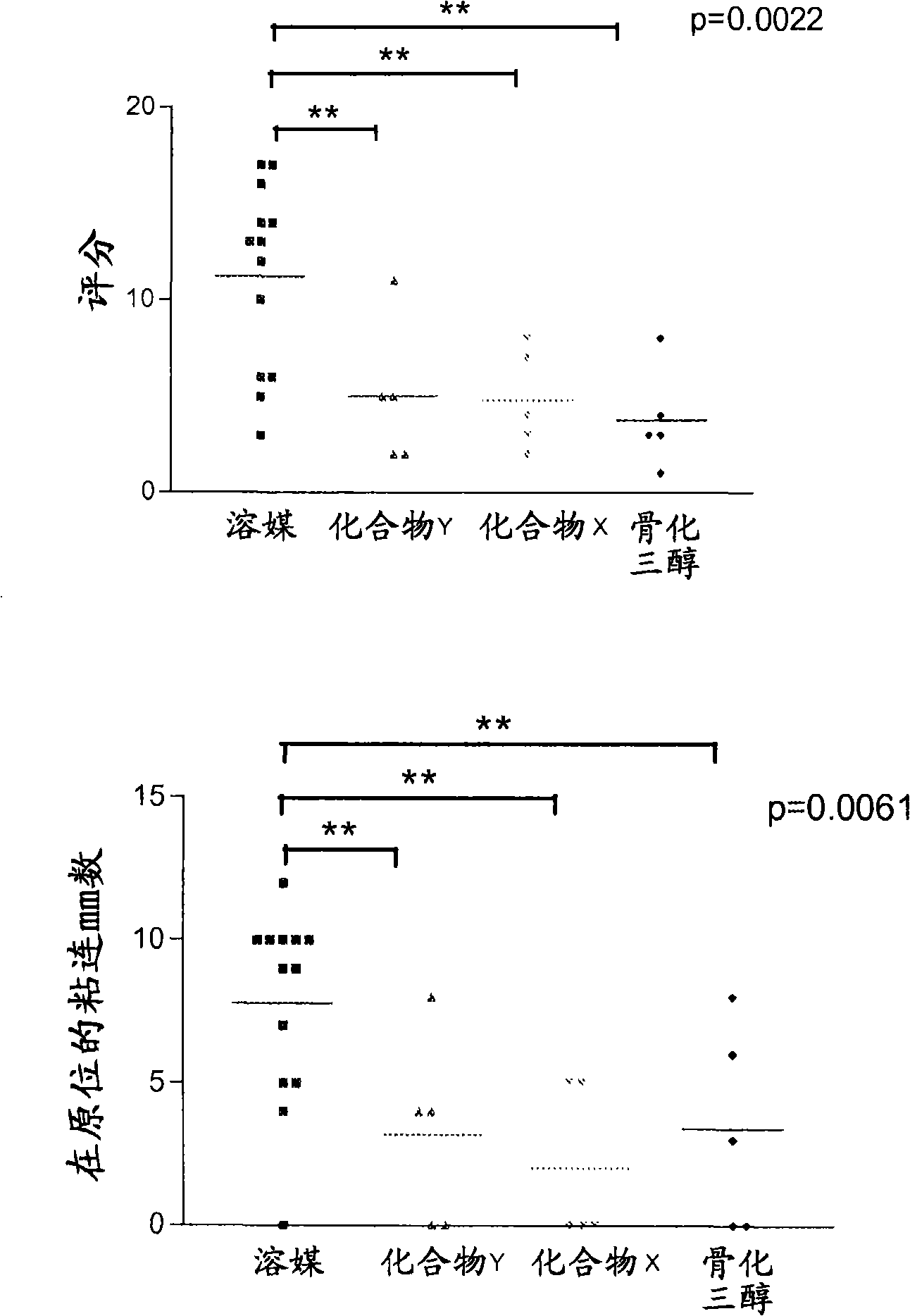
Use of vitamin D compounds for the prevention of adhesions
A compound, vitamin technology, applied to vitamin D compounds in the field of preventing adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 42-50
[0448] Table 1
[0449]
[0450] compound
X
Z
Y
A
B
1,25-dihydroxy-20-cyclopropyl-26,27-hexa
Deutero-19-nor-cholecalciferol
H 2
Oh
Oh
-
-
1,3-Di-O-acetyl-1,25-dihydroxy
-20-cyclopropyl-26, 27-hexadeuterio-19-nor-
H 2
OAc
OAc
-
-
1,25-dihydroxy-20-cyclopropyl-26,27-hexa
Deutero-cholecalciferol
CH 2
Oh
Oh
-
-
1,3-Di-O-acetyl-1,25-dihydroxy
-20-cyclopropyl-26,27-hexadeuterio-cholecalcification
CH 2
OAc
OAc
-
-
1α-fluoro-25-hydroxy-20-cyclopropyl-26,27-
Hexadeuterio-cholecalciferol
CH 2
Oh
F
-
-
3-O-acetyl-1α-fluoro-25-hydroxy-20-
cyclopropyl-cholecalciferol
CH 2
OAc
F
...
Embodiment 1
[0515] Synthesis of 1-α-fluoro-25-hydroxy-16,23E-diene-26,27-dihomo-20-epi-cholecalciferol
[0516] tert-butyl-dimethyl-(4-methylene-3-{2-[7a-methyl-1-(1,4,5-trimethyl-hex-2-enyl)-octahydro- Inden-4-ylidene]-ethylidene}-cyclohexyloxy)-silane
[0517]
[0518] To the stirred 4-methylene-3-{2-[7a-methyl-1-(1,4,5-trimethyl-hex-2-enyl)-octahydro-inden-4-ylidene Base]-ethylidene}-cyclohexanol (100.00g, 0.25mol) in DMF (250mL) was added continuously imidazole (40.80g, 0.6mol) and (tert-butyldimethyl)silyl chloride ( 45.40 g, 0.3 mol). The reaction mixture was stirred at room temperature for 1 hour, diluted with hexane (750 mL), washed with water (500 mL), 1N HCl (500 mL), brine (500 mL) and washed with Na 2 SO 4 dry. The residue (155 g) after evaporation of the solvent was filtered through a silica gel column (500 g, 5% AcOEt in hexane) to give the title compound (115.98 g, 0.23 mol, 92%).
[0519] 1 H-NMR: δ0.04 and 0.08 (2s, 6H), 0.59 (s, 3H), 0.90 (d, 3H, J=6.6Hz), 0.92...
Embodiment 2
[0667] Synthesis of 21-(3-hydroxy-3-methylbutyl)-1,25-dihydroxy-19-nor-cholecalciferol
[0668] [1R-[1α(2E,4E,7E),3aβ,4α,7aα]]-5-[4-[[(1,1-Dimethylethyl)dimethylsilyl]oxy]octahydro -7a-Methyl-1H-inden-1-yl]-2,4,7-diethyl nonatrienedioate
[0669]
[0670] To stirred 3.08g (10.0mmol) of [1R-(1α, 3aβ, 4α, 7aα)]-(1,1-dimethylethyl)dimethyl[[octahydro-7a-methyl-1 -(1-methylvinyl)-1H-inden-4-yl]oxy]silane and 3.92g (40.0mmol) of ethyl propiolate in 20mL of dichloromethane were added 40mL (40.0mmol) of 1.0M A solution of ethyl aluminum dichloride in hexane. The mixture was stirred at room temperature under argon for 24 hours, treated with 981 mg (10 mmol) of ethyl propiolate and 7.5 mL (7.5 mmol) of 1.0 M ethylaluminum dichloride in hexane and stirred for a further 18 hours . The resulting orange-red solution was added in portions to a mixture of 200 mL ethyl acetate and 100 mL of 50% brine, and after the hissing subsided, the organic phase was collected and the aqueous phase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com