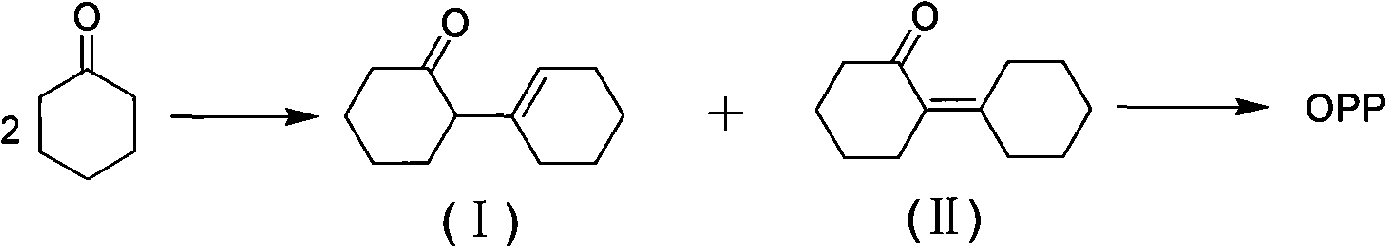Synthetic method of o-phenylphenol
A technology of o-phenylphenol and catalyst, applied in the field of synthetic compounds, can solve the problems of increased cost, increased separation and purification tasks, low industrial value and the like, and achieves the effects of high production efficiency, improved selectivity, and convenient recovery.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Dilute 50g with Na 2 CO 3 Add the modified 1% Pt / C catalyst and 500g of cyclohexanone dimer together into the reactor, fill it with nitrogen for replacement 3 times, heat it to 370-380°C under stirring, and release the hydrogen generated by the reaction in time to maintain the pressure At 1.0~1.2MPa. Sampling analysis, when the o-cyclohexylphenol content in the reaction solution is less than 2%, the reaction is stopped. The reaction liquid was cooled to 70° C. and filtered to obtain a total of 442 g of filtrate. Gas chromatography analysis showed that the content of o-phenylphenol in the reaction liquid was 90.6%, and the conversion rate of cyclohexanone dimer was 100%.
Embodiment 2
[0020] 30g with K 2 CO 3 Add the modified 3% Pt / C catalyst and 500g of cyclohexanone dimer together into the reactor, fill it with nitrogen for replacement 3 times, heat it to 390-400°C under stirring, release the hydrogen generated by the reaction in time to maintain the pressure 1.9-2.0MPa. Sampling analysis, when the o-cyclohexylphenol content in the reaction solution is less than 2%, the reaction is stopped. The reaction solution was cooled to 60° C. and filtered to obtain a total of 449 g of filtrate. Gas chromatography analysis showed that the o-phenylphenol content in the reaction solution was 91.3%, and the conversion rate of cyclohexanone dimer was 100%.
Embodiment 3
[0022] 20g with CaCO 3 Add the modified 5% Pt / C catalyst and 500g of cyclohexanone dimer together into the reactor, fill it with nitrogen for replacement 3 times, heat it to 320-330°C under stirring, release the hydrogen generated by the reaction in time to maintain the pressure It is 0.6-0.7MPa. Sampling analysis, when the o-cyclohexylphenol content in the reaction solution is less than 2%, the reaction is stopped. The reaction solution was cooled to below 65° C. and filtered to obtain a total of 451 g of the filtrate. Gas chromatography analysis showed that the content of o-phenylphenol in the reaction solution was 90%, and the conversion rate of cyclohexanone dimer was 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com