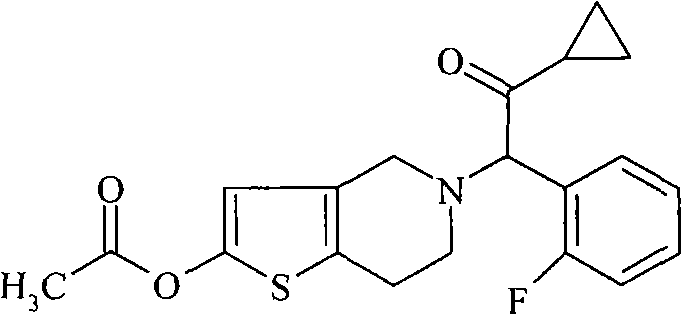
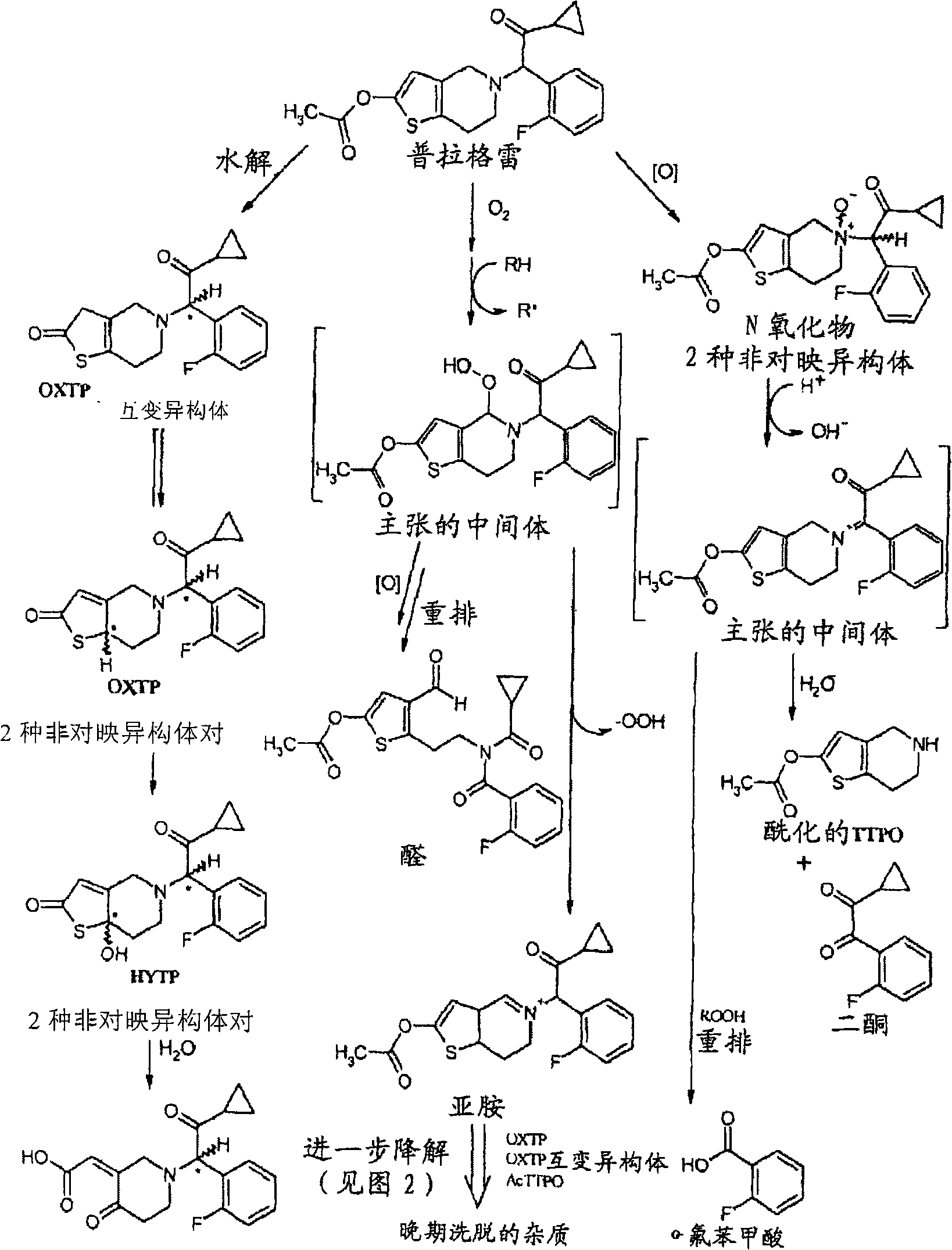
An article of manufacture for prasugrel
A product and moisture technology, applied in the field of products of thienopyridine platelet aggregation inhibitor, can solve the problems of low oxygen concentration, difficult application and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Prasugrel HCl salt (10.98 mg equivalent to 10 mg base), mannitol, hydroxypropyl methylcellulose, croscarmellose sodium, microcrystalline cellulose, and magnesium stearate were mixed and rolled Machine compaction to produce granules. To the resulting granulate is added additional croscarmellose sodium, microcrystalline cellulose and magnesium stearate, and the material is mixed and compressed to form tablets weighing from about 175 mg to about 250 mg. Opadry The beige coating mixture was added to the water and sprayed onto the tablets in a side vented coating pan.
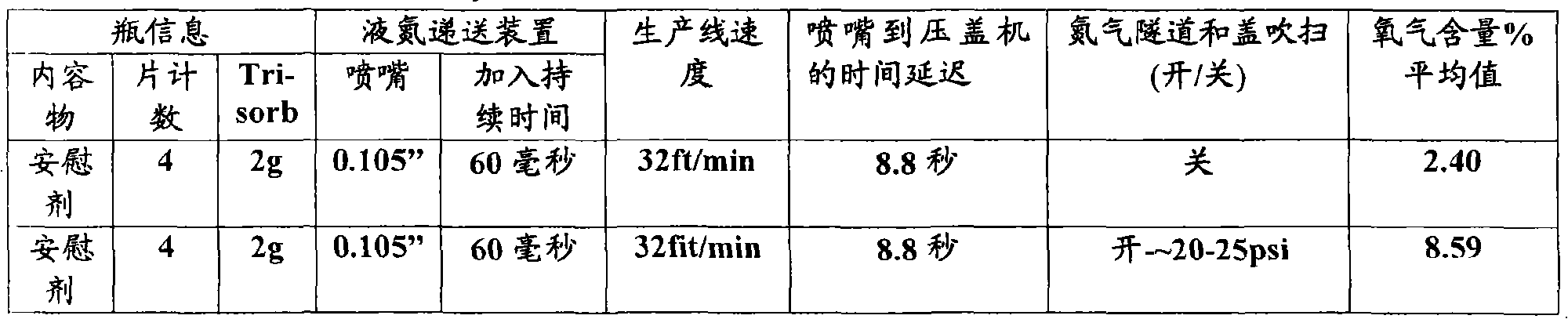
[0109] The tablets are then packaged with a molecular sieve desiccant in a barrier bottle, then inertized with a liquefied gas such as liquid nitrogen, capped and then sealed by methods known to those skilled in the art. The table below shows some representative parameters that have been successfully used to inert bottles with liquid nitrogen.
[0110] Table 5 - Liquid Nitrogen Inertization - Prasugrel 10...
Embodiment 2
[0120] Prasugrel HCl salt (5.49 mg equivalent to 5 mg base), mannitol, hydroxypropyl methylcellulose, croscarmellose sodium, microcrystalline cellulose and magnesium stearate were mixed and rolled Machine compaction to produce granules. To the resulting granulate is added additional croscarmellose sodium, microcrystalline cellulose and magnesium stearate, and the material is mixed and compressed to form tablets weighing from about 125 mg to about 250 mg. Opadry The beige film coating mixture was added to the water and sprayed onto the tablets in a side vented coating pan.
[0121] The tablets are then packaged with a molecular sieve desiccant in a barrier bottle and then inertized with a liquefied gas such as liquid nitrogen, capped and then sealed by methods known to those skilled in the art. The table below shows some representative parameters for bottles that have been successfully inertized with liquid nitrogen.
[0122] Table 7 - Liquid Nitrogen Inertization - Prasugr...
Embodiment 3
[0132] Mix prasugrel HCl salt (8.24 mg equivalent to 7.5 mg base), mannitol, hydroxypropyl methylcellulose, croscarmellose sodium, microcrystalline cellulose, and magnesium stearate, then roll The press crushes to produce pellets. To the resulting granulate is added additional croscarmellose sodium, microcrystalline cellulose and magnesium stearate, and the material is mixed and compressed to form tablets weighing from about 125 mg to about 250 mg. Opadry The beige film coating mix was added to the water and then sprayed onto the tablets in the side vent coating process.
[0133] The tablets are then packaged with a molecular sieve desiccant in a barrier bottle, then inertized with a liquefied gas such as liquid nitrogen, capped and then sealed by methods known to those skilled in the art. See Tables 4 and 5 for examples of settings that might be used to inert these barrier bottles with liquid nitrogen. The tablets, caplets or capsules are then placed in boxes for storage ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com