Antibodies and immunoconjugates and uses therefor
An immunoconjugate, antibody technology, applied in the direction of antibodies, anti-animal/human immunoglobulin, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve problems such as low efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
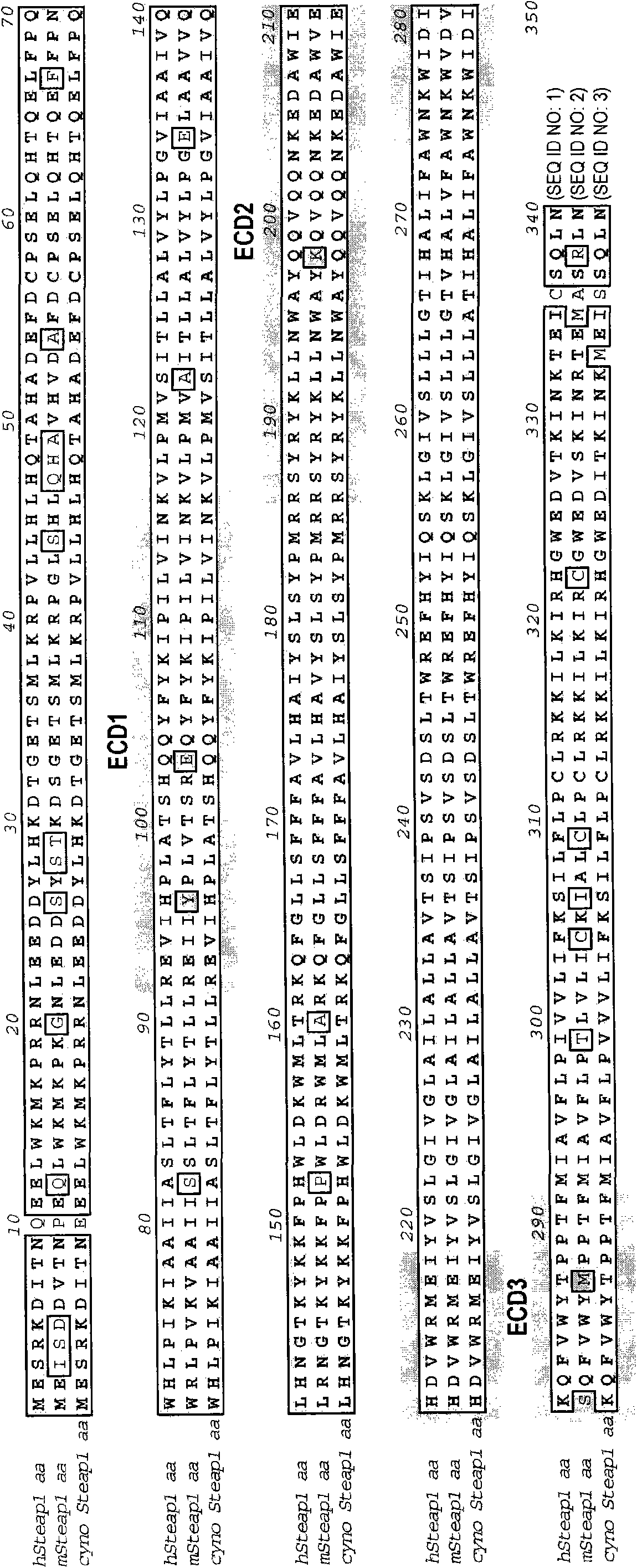
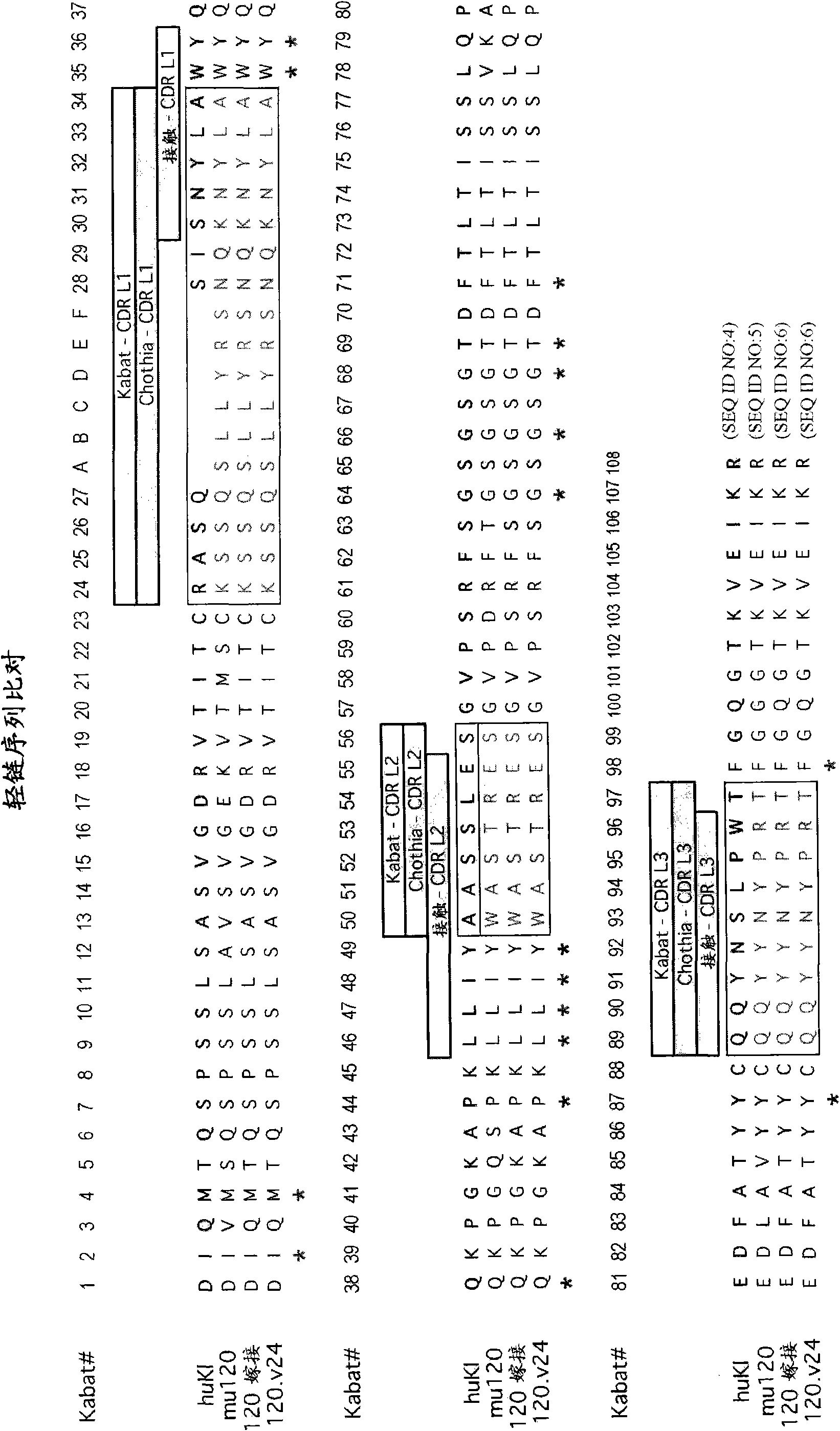
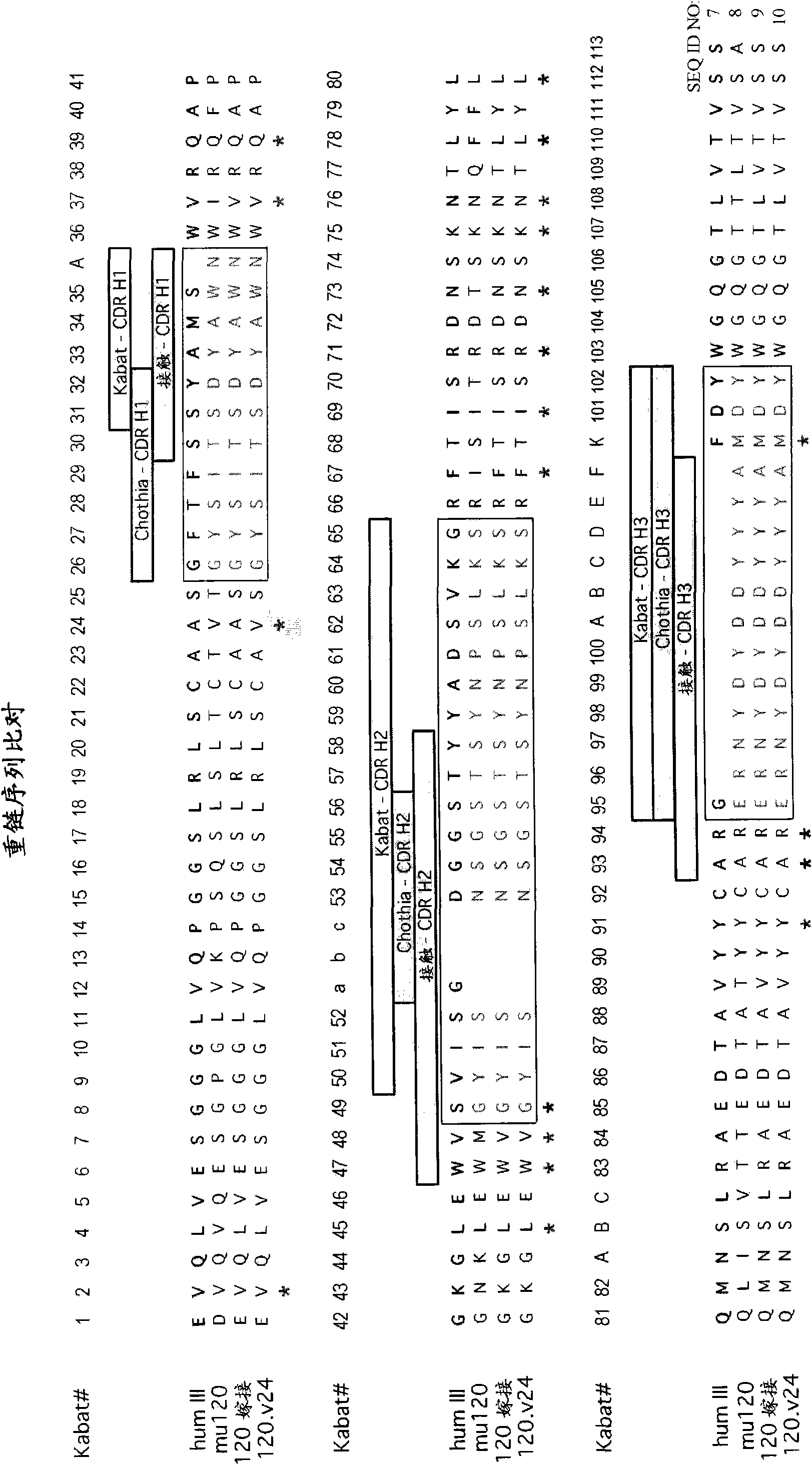
[0820] Example 1: Preparation of humanized anti-STEAP-1 antibody
[0821] Nucleic acid molecules encoding amino acid sequence variants of antibodies, antibody fragments, VL domains or VH domains are prepared by a variety of methods known in the art. These methods include, but are not limited to, isolation from natural sources (in the case of naturally-occurring amino acid sequence variants) or by performing variant or non-variant versions of antibodies, antibody fragments, VL domains or VH domains produced earlier. Oligonucleotide-mediated mutagenesis (or site-directed mutagenesis), PCR mutagenesis and cassette mutagenesis. For example, the method of Kunkel can be used to create a library by targeting VL accessible amino acid positions in the VH and optionally in one or more CDRs and making amino acid substitutions with variant amino acids. See, eg, Kunkel et al., Methods Enzymol. (1987), 154:367-382 and the Examples therein. The generation of randomization sequences is also...
Embodiment 2
[0884] Example 2: Characterization of Anti-STEAP-1 Antibodies
[0885]Anti-STEAP-1 antibodies (naked antibodies and antibody drug conjugates disclosed herein) were characterized according to standard methods.
[0886] ELISA-Based Assay: Anti-STEAP-1 antibody screening by ELISA was performed as follows, with all incubations at room temperature. The test plate (Nunc Immunoplate) was coated with purified STEAP-1 in 50nM sodium carbonate buffer (pH9.6) for 2 hours, then blocked with 0.5% bovine serum albumin in phosphate buffered saline (PBS) for 30 minutes, Then wash 4 times with PBS (PBST) containing 0.05% Tween 20. Test antibody supernatants were added and incubated with shaking for 2 hours, then washed 4 times with PBST. By adding 100 μl / well solution (containing 10 mg o-phenylenediamine dihydrochloride (Sigma, #P8287) and 10 μl 30% hydrogen peroxide solution in 25 ml phosphate citrate buffer (pH 5.0)) and incubating for 15 minutes To color the plate. The reaction was stop...
Embodiment 3
[0889] Example 3: Generation of anti-STEAP-1 antibody-drug conjugates
[0890] Generation of anti-STEAP-1 auristatin ADCs - Anti-STEAP-1 ADCs were generated by coupling anti-STEAP-1 antibody murine 120.545, 120 chimera, 120 graft, and humanized 120 framework variants to the following drug-linker modules: spp-DM1, smcc-DM1, MC-vc-PAB-MMAE, MC-vc-PAB-MMAF, MC-MMAE, MC-MMAF, vc-MMAE, and vc-MMAF, the drugs and linker modules and methods of attachment disclosed WO2004 / 010957, published February 5, 2004; WO2006 / 034488, published September 9, 2005; and Doronina, S.O. et al., Nature Biotechnol. 21:778-784 (2003) (each reference The literature is included in this article in its entirety by reference). Prior to conjugation, antibodies were partially reduced with TCEP using standard methods following the methodology described in WO 2004 / 010957. The partially reduced antibody was coupled to the drug-linker moiety described above using standard methods following the methodology describe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com