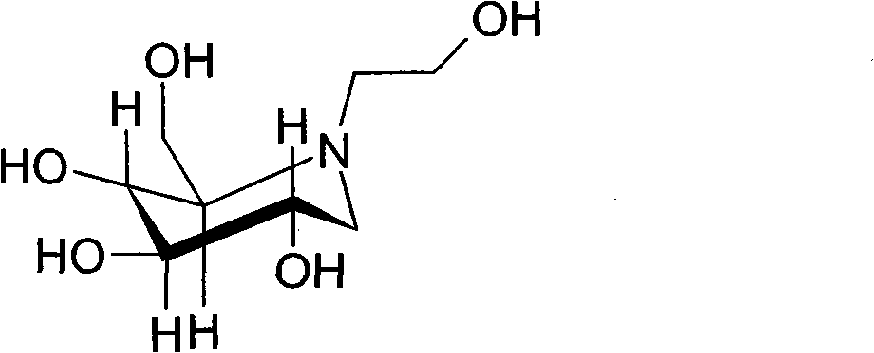
Migltol microcapsule tablet and preparation method thereof
A technology of miglitol and microcapsule tablets, which is applied in the field of medicine, can solve the problems of affecting the stability of drugs, the decrease of dissolution rate, the increase of substances, etc., and achieve the effects of benefiting quality control, improving stability and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
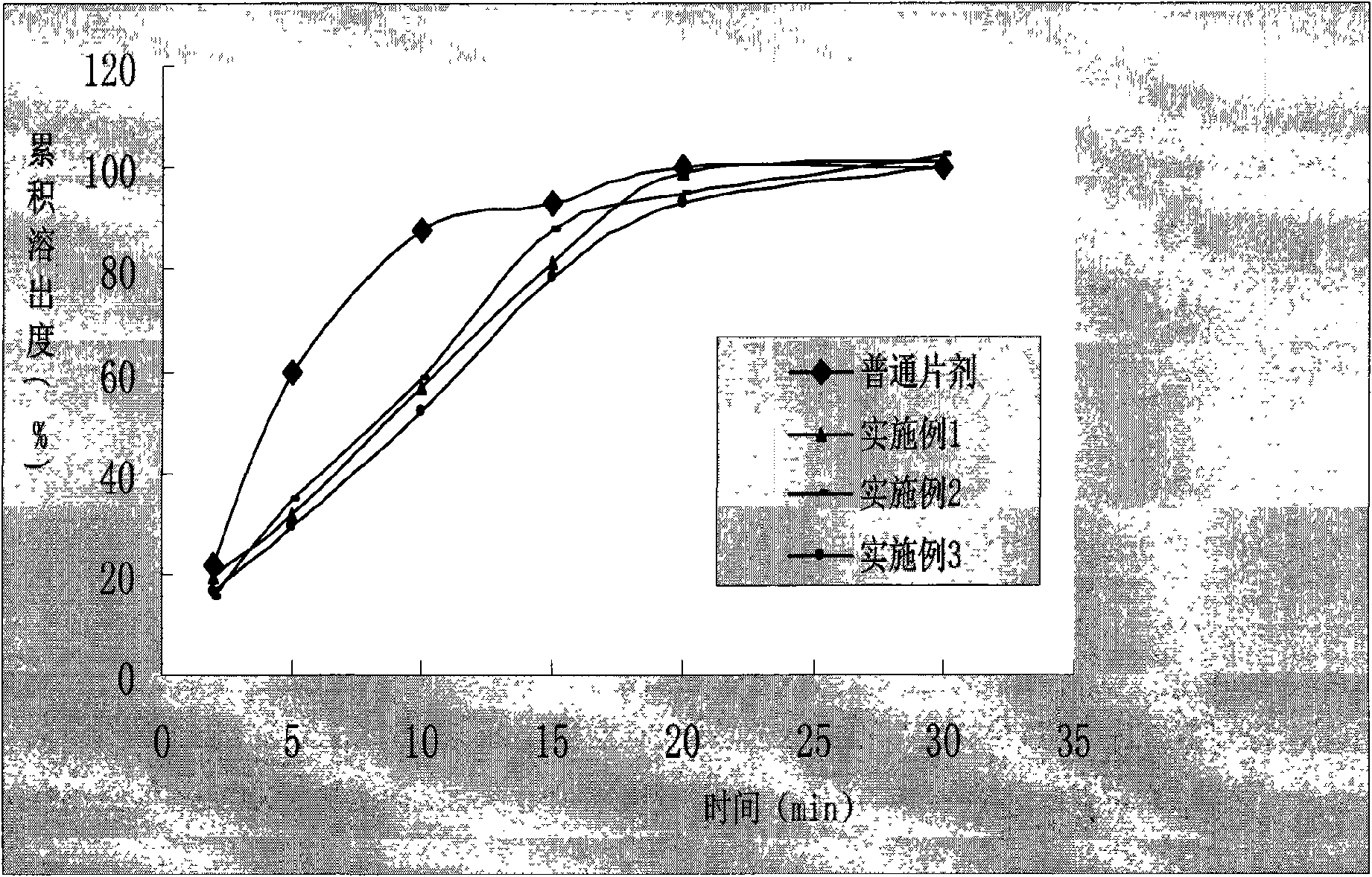
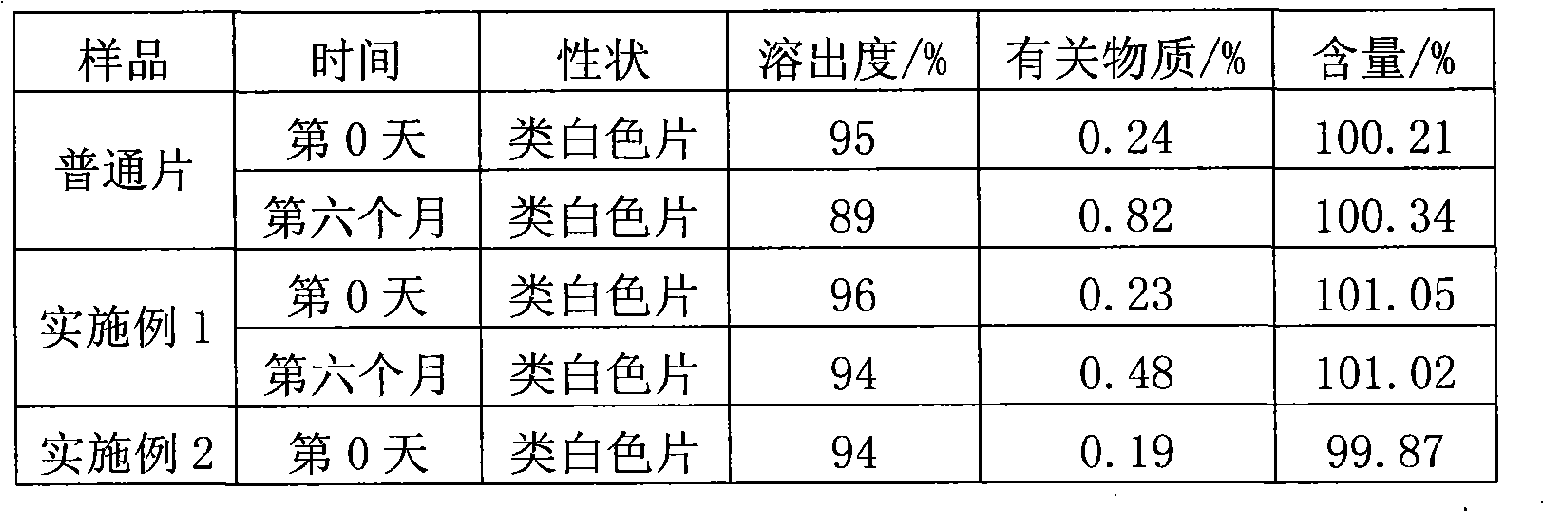
Image
Examples
Embodiment 1
[0034] Microcapsule preparation of Miglitol of the present invention
[0035] Name of raw material Amount / g
[0036]
[0037] Miglitol 50
[0038] starch 10
[0039] Type IV Acrylic 30
[0040] MCC 50
[0041] CMS-Na3
[0042] 70% ethanol water solution
[0043]
[0044] A total of 1000 pieces were made
[0045] Preparation Process:
[0046] ① Pass the prescribed amount of Miglitol through a 60-120 mesh sieve, place it in a fluidized bed, and pass hot air into it to suspend and fluidize it. The temperature of the hot air is 45-55°C;
[0047]② Dissolve starch and type IV acrylic resin with an appropriate amount of 70% ethanol aqueous solution to make a 15% capsule material solution, and the capsule material solution is atomized through the nozzle of the fluidized bed and continuously added to the fluidized bed, where the atomization pressure is 0.25-0.30Mpa , the spraying speed is 50...
Embodiment 2
[0050] Microcapsule preparation of Miglitol of the present invention
[0051] Name of raw material Amount / g
[0052]
[0053] Miglitol 50
[0054] Polyethylene glycol 15
[0055] HPMC 20
[0056] Lactose 60
[0057] CMC-Na4
[0058] 70% ethanol water solution
[0059]
[0060] A total of 1000 pieces were made
[0061] Preparation Process:
[0062] ① Pass the prescribed amount of Miglitol through a 60-120 mesh sieve, place it in a fluidized bed, and pass hot air into it to suspend and fluidize it. The temperature of the hot air is 45-55°C;
[0063] ② Dissolve HPMC and polyethylene glycol with an appropriate amount of 70% ethanol aqueous solution to make a 15% capsule material solution, and the capsule material solution is atomized through the nozzle of the fluidized bed and continuously added to the fluidized bed, wherein the atomization pressure is 0.25-0.30Mpa , the spra...
Embodiment 3
[0066] Microcapsule preparation of Miglitol of the present invention
[0067] Name of raw material Amount / g
[0068]
[0069] Miglitol 50
[0070] PvP 35
[0071] Calcium hydrogen phosphate 20
[0072] MCC 40
[0073] Copovidone S-630 4
[0074] 70% ethanol water solution
[0075]
[0076] A total of 1000 pieces were made
[0077] Preparation Process:
[0078] ① Pass the prescribed amount of Miglitol through a 60-120 mesh sieve, place it in a fluidized bed, and pass hot air into it to suspend and fluidize it. The temperature of the hot air is 45-55°C;
[0079] ②Dissolve PVP with an appropriate amount of 70% ethanol aqueous solution to make a 20% capsule material solution. The capsule material solution is atomized and continuously added to the fluidized bed through the nozzle of the fluidized bed, wherein the atomization pressure is 0.25-0.30Mpa, and the spraying speed is 50 ~...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com