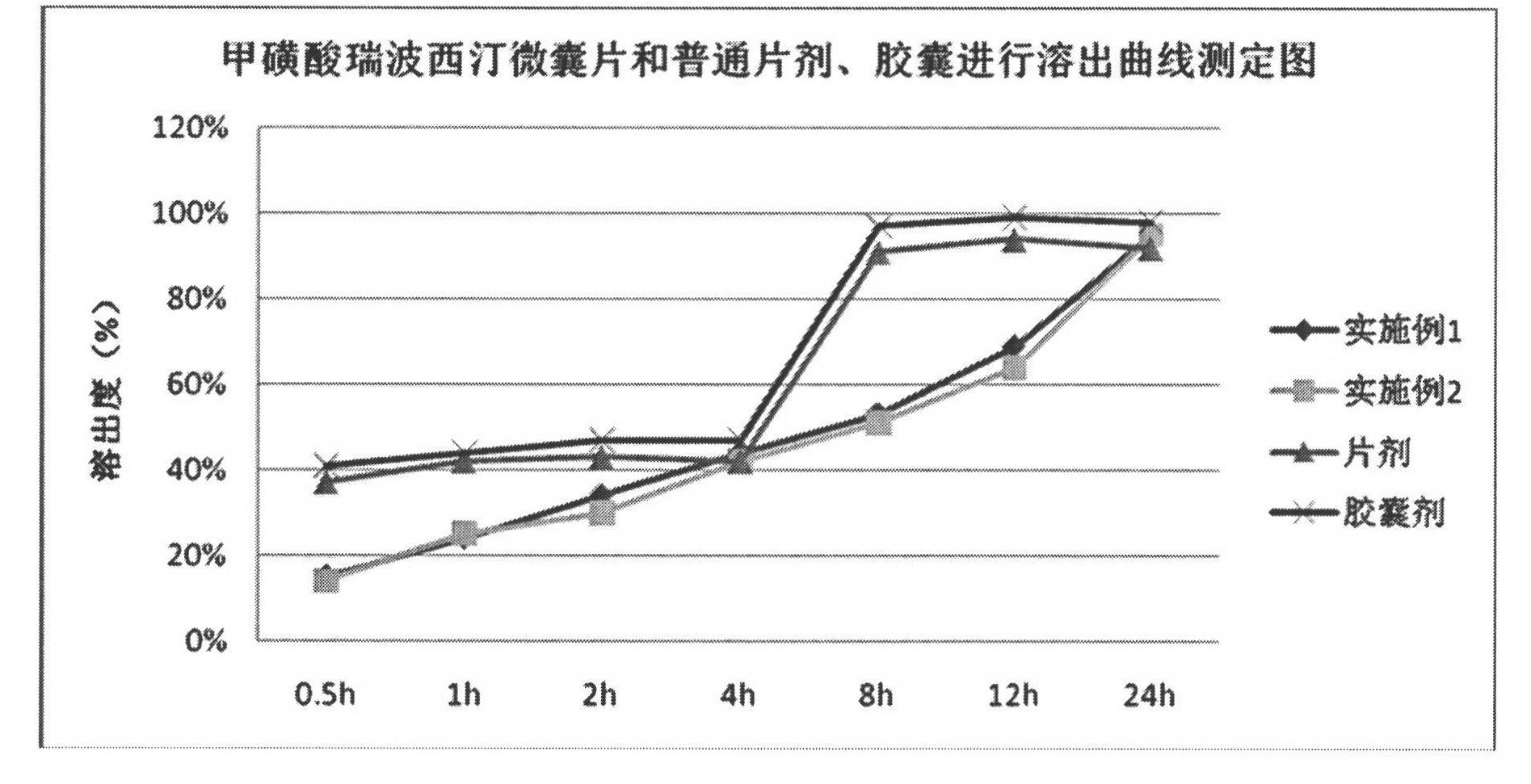
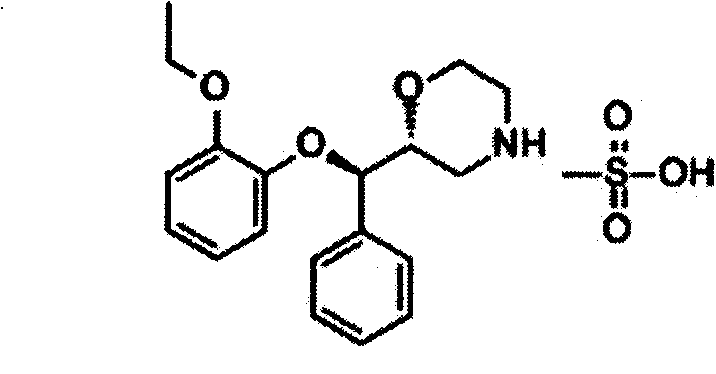
Reboxetine mesylate microencapsule tablet and preparation method thereof
A technology of reboxetine mesylate and microcapsules, which is applied in the field of reboxetine microcapsules and its preparation, can solve the problems of increasing adverse drug reactions, increasing blood drug concentration, adverse reactions, etc., and achieve improved bioavailability , enhance the therapeutic effect, reduce the effect of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] prescription:
[0035]
[0036] Pass the prescription amount of reboxetine mesylate through an 80 mesh sieve, place it in a fluidized bed, and pass in hot air at a temperature of 45°C. Add prescription amount of talc; combine gum arabic and dimethyl methacrylate The amino ethyl ester is made into a 15% capsule material solution with an appropriate amount of 70% ethanol aqueous solution for later use. The capsule material solution is atomized through a fluidized bed nozzle and sprayed on the surface of the fine powder. The spray atomization pressure is 0.35Mpa and the spray speed is 45rpm, continuous ventilation and drying, after coating, stop heating, cool to 30°C, discharge, and obtain reboxetine mesylate microcapsules; combine the prepared reboxetine mesylate microcapsules with the prescribed amount of microcrystals Cellulose, lactose, sodium starch glycolate, and magnesium stearate are mixed uniformly and directly compressed to obtain a tablet.
Embodiment 2
[0038] prescription:
[0039]
[0040] Pass the prescription amount of Reboxetine mesylate through an 80-mesh sieve, place it in a fluidized bed, pass in hot air at a temperature of 45°C, and add a prescription amount of magnesium stearate; combine the polyvinyl alcohol and hypromellose The base cellulose is made into a 12% capsule material solution with an appropriate amount of 70% ethanol aqueous solution for use. The capsule material solution is atomized through a fluidized bed nozzle and sprayed on the surface of the fine powder. The spray atomization pressure is 0.40Mpa, and the spray speed is 45rpm, continuous ventilation and drying, after the coating is finished, stop heating, cool to 30℃, discharge, and obtain reboxetine mesylate microcapsules; combine the prepared reboxetine mesylate microcapsules with the prescription amount of hydrogen phosphate Calcium, starch, sodium starch glycolate, and magnesium stearate are mixed uniformly and directly compressed to obtain a tabl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com