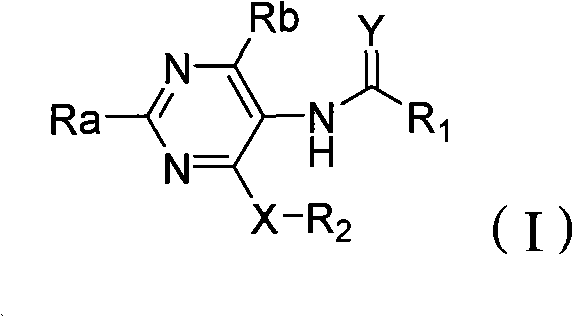
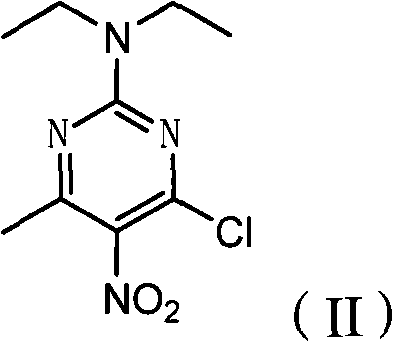
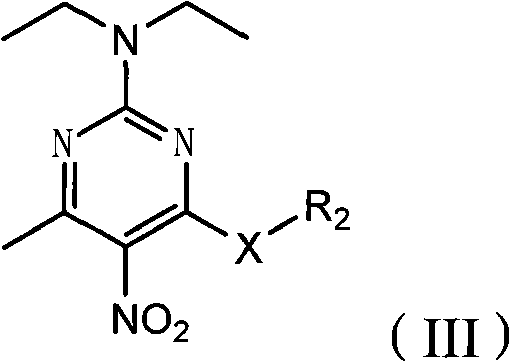
Poly-substituted miazines compound, preparation method and application thereof
A compound and multi-substitution technology, which is applied in the field of multi-substituted pyrimidine compounds and its preparation, can solve the problems of plant virus disease prevention and control, plants without immune metabolism system, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] 1-(2-(Diethylamine)-4-methyl-6-(4-phenoxyphenoxy)-5-pyrimidinyl)-3-(4-methoxyphenyl)thiourea
[0090]
[0091] Its preparation process is as follows:
[0092] Step A: N,N-Diethyl-4-methyl-5-nitro-6-(4-phenoxyphenoxy)pyrimidin-2-amine
[0093] Dissolve 0.4g of 4-chloro-N,N-diethyl-6-methyl-5-nitropyrimidin-2-amine in 20mL of anhydrous benzene, add 0.37g of 4-phenoxyphenol, 0.1g of sodium hydride , stirred at room temperature for 12 hours, evaporated to remove benzene, washed with water and dried to obtain 0.55 g of the expected product with a yield of 85%.
[0094] Step B: N 2 , N 2 -Diethyl-4-methyl-6-(4-phenoxyphenoxy)pyrimidine-2,5-diamine
[0095] Dissolve 2 g of the product obtained in step A in 70 mL of ethanol, and reduce it by hydrogenation at normal temperature and pressure under the catalyst of 0.5 g of Raney Ni. After reacting for 30 hours, the catalyst was filtered off and the solvent was distilled off to obtain 1.85 g of the expected product with a y...
Embodiment 2
[0103] 1-(4-(Diphenyl-4-oxo)-2-(diethylamine)-6-methyl-5-pyrimidinyl)-3-o-tolylthiourea
[0104]
[0105] The preparation steps are basically the same as in Example 1. 4-Phenylphenol was used as a reactant in Step A and 1-isothiocyanato-2-methylbenzene was used as a reactant in Step C.
[0106] The final product is a white solid:
[0107] 1 H NMR (CDCl 3 )(ppm): 7.19~7.61(15H, m), 3.36~3.59(4H, m), 2.38(6H, m), 1.02~1.16(6H, m)
[0108] MS: 498.3 (M-1 + ), 520.3 (M-1+Na + )
[0109] IR: 2960, 1608, 1403, 1210
Embodiment 3
[0111] 1-(4-(Diphenyl-4-oxo-)-2-(diethylamine)-6-methyl-5-pyrimidinyl)-3-(4-methoxyphenyl)thiourea
[0112]
[0113] The preparation steps are basically the same as in Example 2. In Step C 1-isothiocyanato-4-methoxybenzene is used as a reactant.
[0114] The final product is a white solid:
[0115] 1 H NMR (CDCl 3 )(ppm): 6.91~7.61(13H, m), 3.80(3H, s), 3.36~3.58(4H, dm), 2.36(3H, s), 1.01~1.17(6H, m)
[0116] MS: 512.1
[0117] IR: 2960, 1608, 1519, 1249, 842, 759, 693
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com