Method for converting aconite diester-type alkaloids into ester-type alkaloids
A technology of diester-type alkaloids and lipid-type alkaloids, which is applied in organic chemistry and other fields, and can solve problems such as high equipment requirements, low solubility, and reduced conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
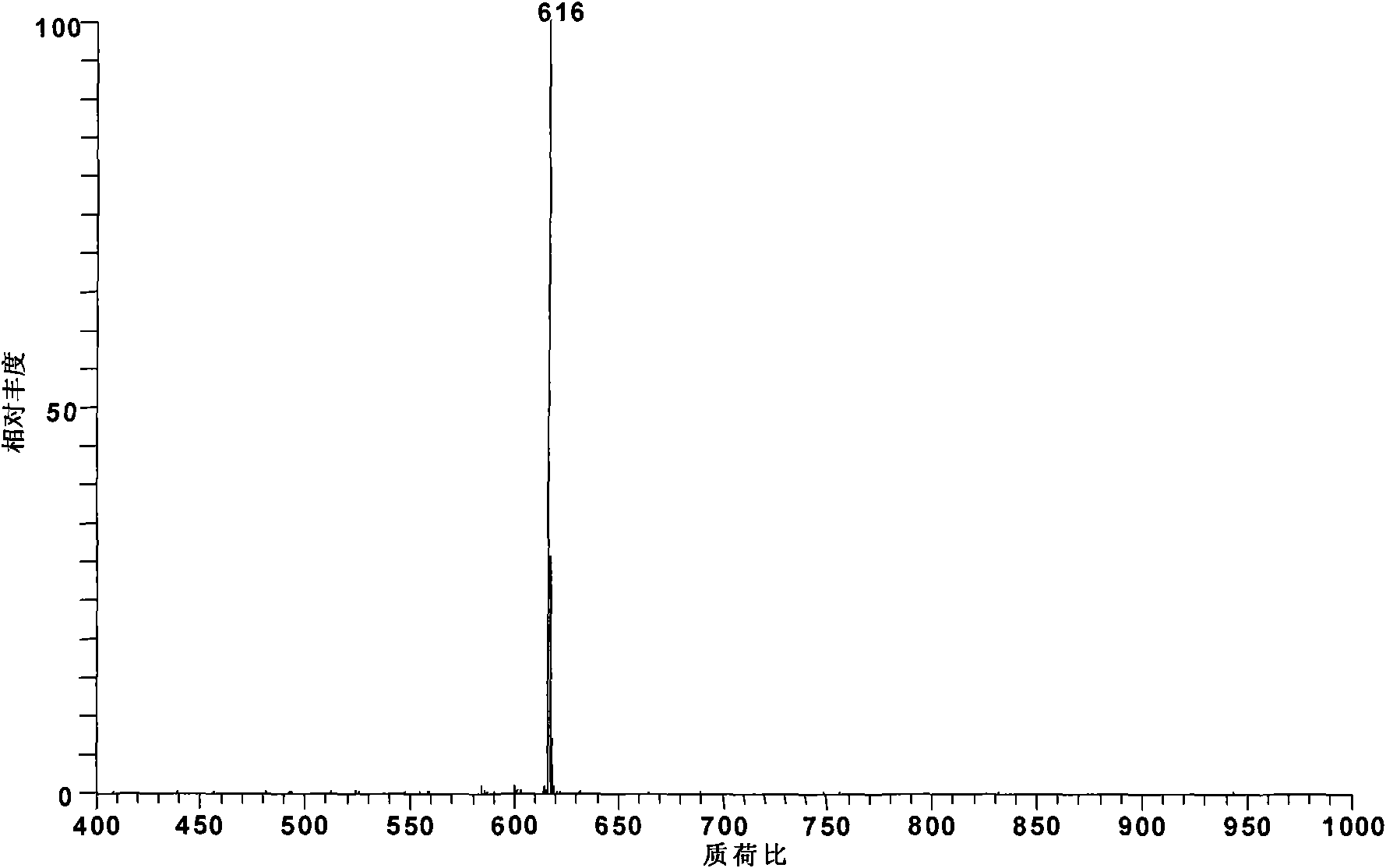
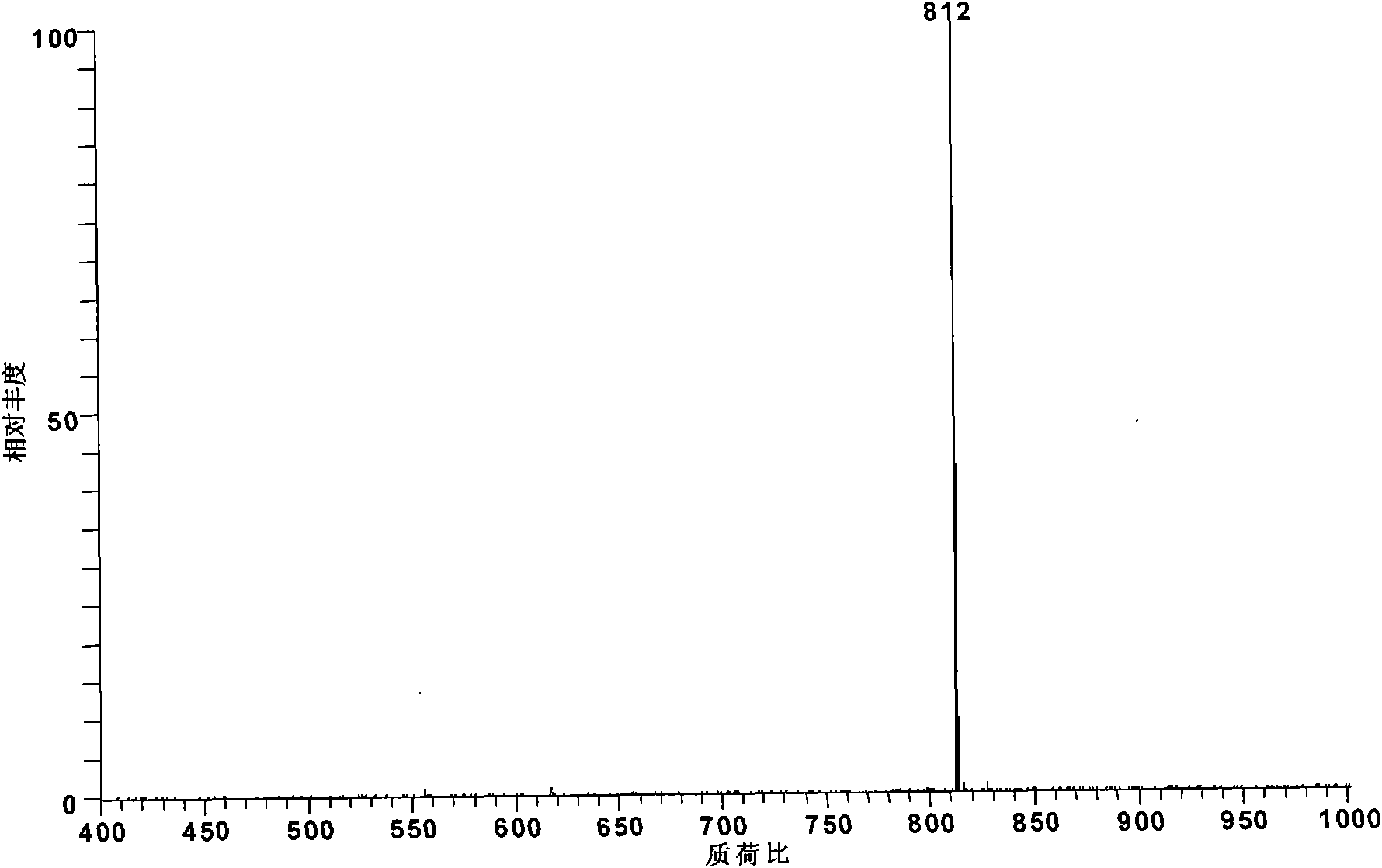
[0020] Add 1 mg of aconitine standard substance into 50 mL of toluene, and at this time, toluene has submerged the hypoconitine standard substance, then add 4.2 mg of palmitic acid and 0.25 mL of pyridine, and heat to reflux in a water bath at 95 ° C for 4 hours to obtain Lipid alkaloid: 8-palmitoyl-benzoyl aconitine (m / z 812).
[0021] The obtained reaction product was diluted 100 times with methanol and detected by electrospray mass spectrometry. According to the relative abundance ratio equal to the molar concentration ratio in electrospray mass spectrometry, the conversion rate of hypoaconitine was 97%. The alkaloid components generated by the reaction are: 8-palmitoyl-benzoyl aconitine (m / z812).
Embodiment 2
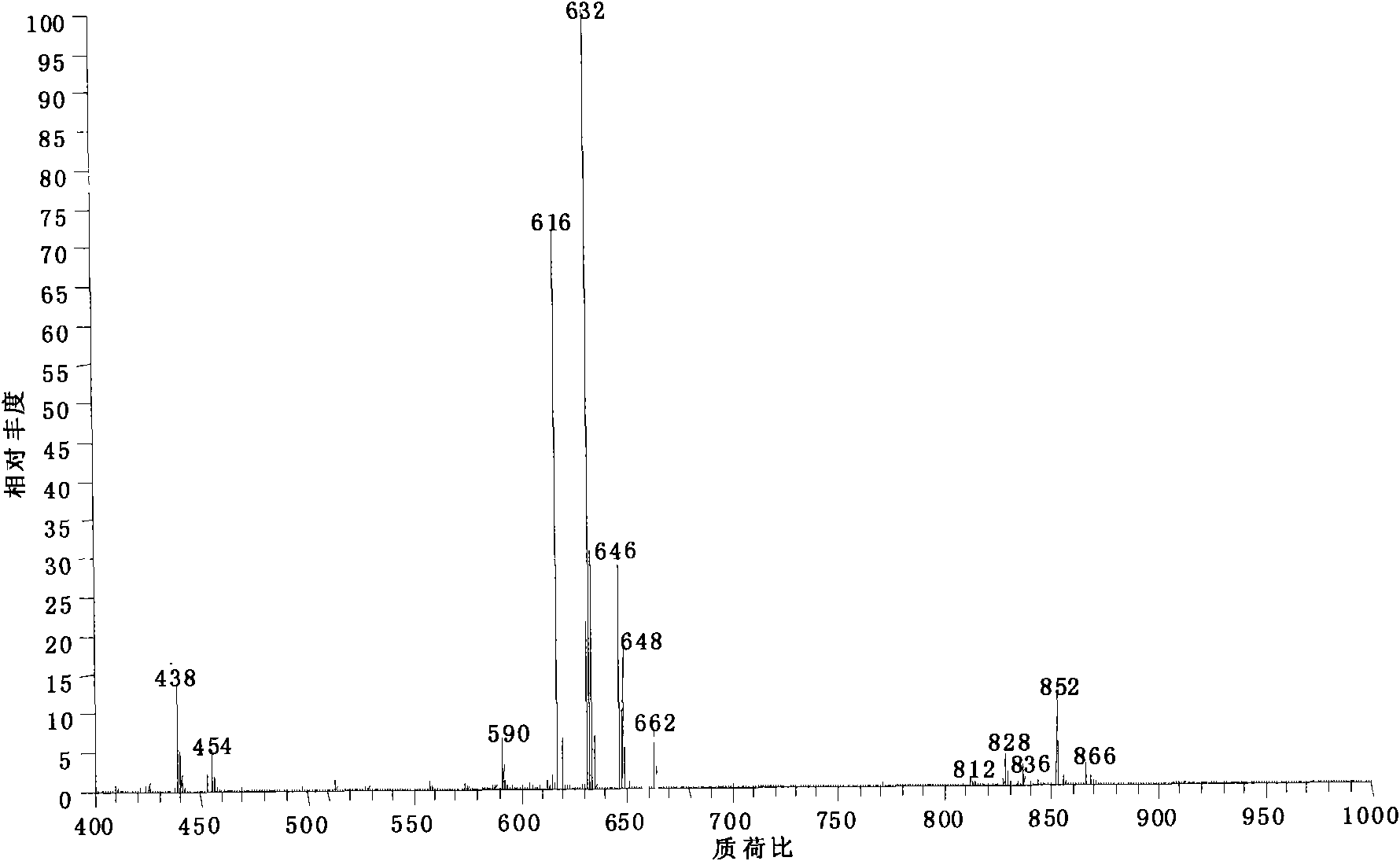
[0023] Add 1 mg of aconitine standard substance into 100 mL of toluene, at this time, the toluene has submerged the hypoconitine standard substance, then add 22 mg of oleic acid and 1 mL of pyridine into toluene, and heat to reflux in a 97°C water bath for 8 hours, Lipid alkaloid: 8-oleoyl-benzoyl aconitine (m / z 838) was obtained.
[0024] The obtained reaction product was diluted 100 times with methanol and then detected by electrospray mass spectrometry. According to its relative abundance ratio equal to molar concentration ratio in electrospray mass spectrometry, the conversion rate of hypoaconitine was 95%. The alkaloid component generated by the reaction is: 8-oleoyl-benzoyl aconitine (m / z838).
Embodiment 3
[0026] Add 1mg of aconitine standard substance into 100mL of toluene, and then add 40mg of linoleic acid and 5mL of pyridine to toluene, and heat to reflux in a water bath at 100°C for 12 hours , to obtain lipid alkaloid: 8-linoleoyl-benzoyl aconitine (m / z 836).
[0027] The resulting reaction product was diluted 100 times with methanol and then detected by electrospray mass spectrometry. According to its relative abundance ratio equal to the molar concentration ratio in electrospray mass spectrometry, the conversion rate of hypoaconitine was 94%. The alkaloid component generated by the reaction is: 8-linoleoyl-benzoyl aconitine (m / z836).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com