Ruthenium-based catalytic complexes and the use of such complexes for olefin metathesis
A compound and alkyl technology, applied in the field of new ruthenium-based catalytic complexes and their synthesis, can solve problems such as difficult recycling of complexes, residual pollution of toxic metals, and harmful synthesis of high value-added products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
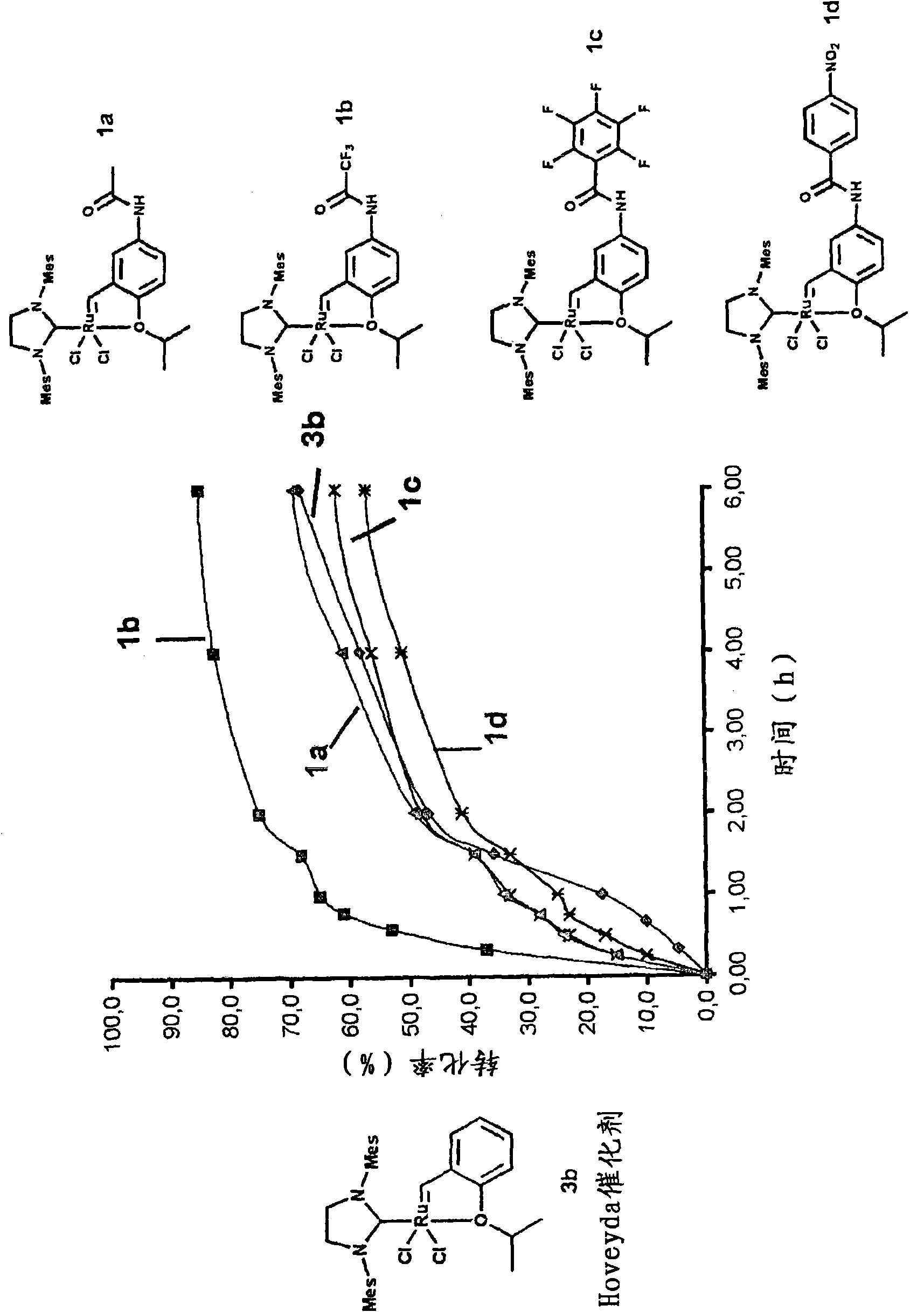
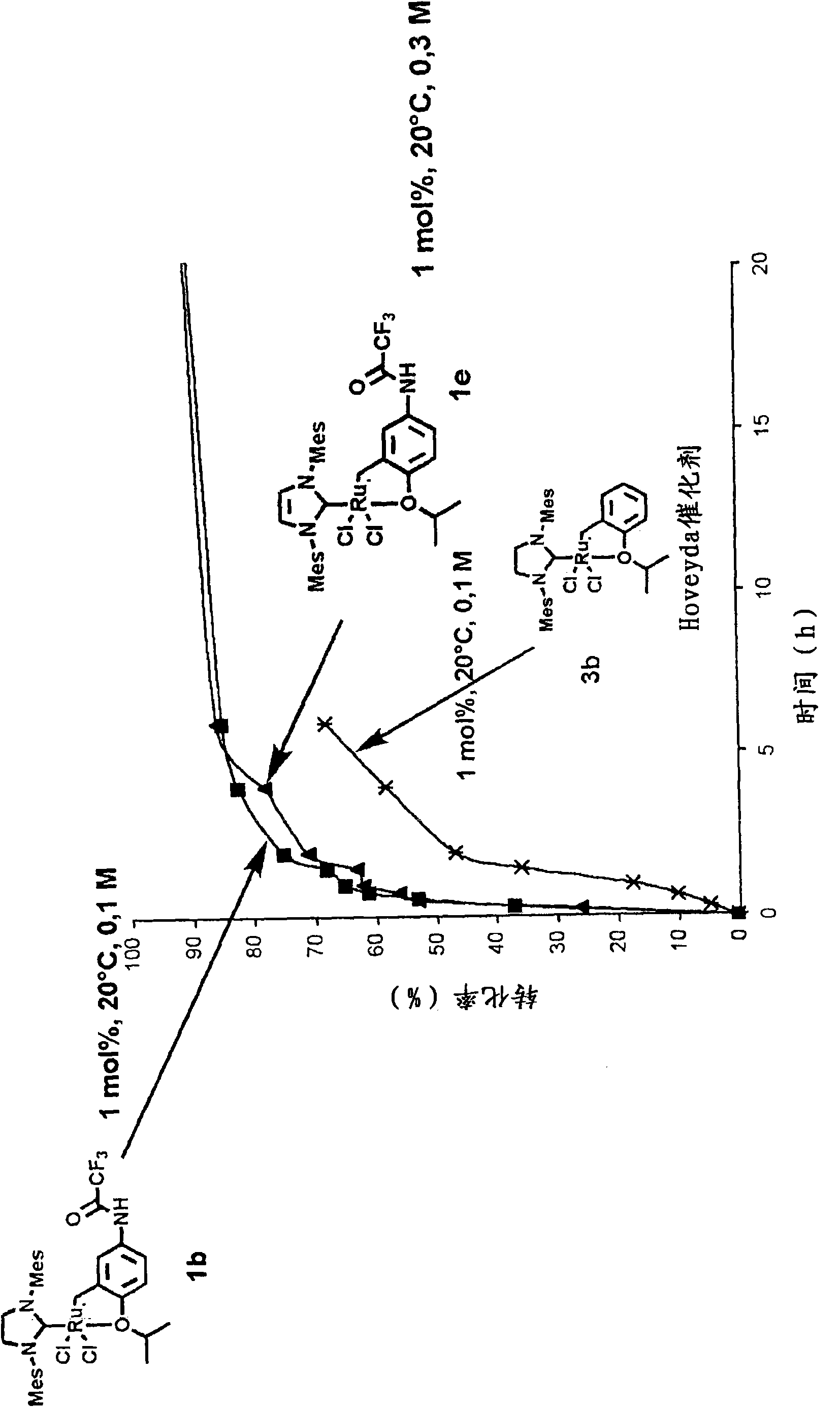
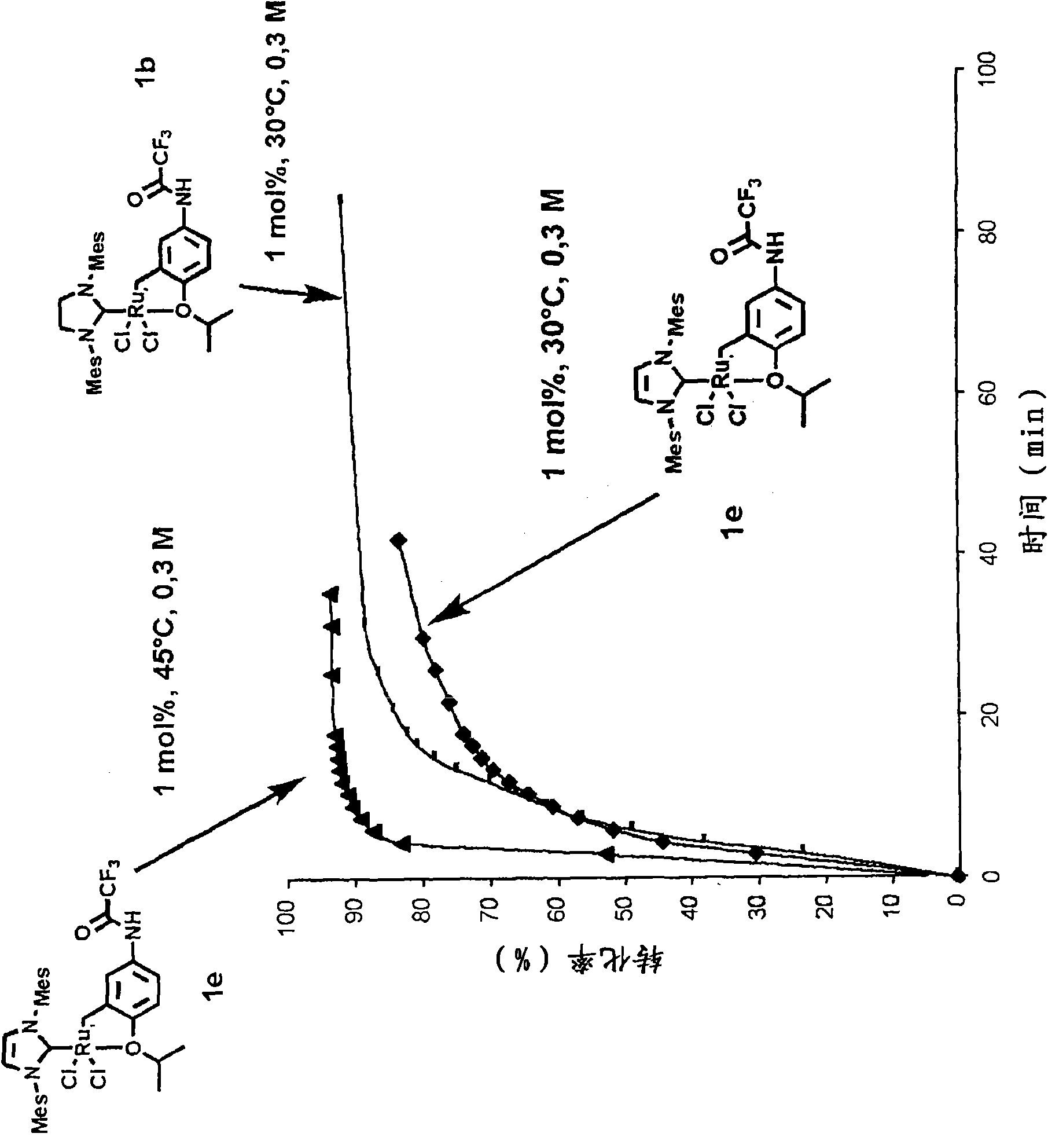
[0105] First, the synthesis of various examples of the complexes of the present invention will be described below.
[0106] Complexes 1a, 1b, 1c, 1d, 1e and 1f of the present invention were obtained from functionalized aniline 5 in two steps.
[0107] The method for synthesizing the functionalized aniline 5 from p-nitrophenol in 4 steps is described in the article "Activated pyridinium-tagged ruthenium complex as efficient catalyst for Ring-Closing Metathesis (activated pyridinium-tagged ruthenium complex as a ring-closing metathesis effective catalyst)" D. Rix, H. Clavier, Y. Coutard, L. Gulajski, K. Grela*, M. Mauduit*, J. Organomet. Chem., 2006, 691, 5397-5405.
[0108] The following roadmap outlines this two-step synthesis:
[0109]
[0110] Step 1: Synthesis of amides 6a, 6b, 6c, 6d, 6f, 9a, 9b, 10a and 10b from 4-isopropylhydrogen-3-vinylaniline 5
[0111] According to the general procedure, 4-isopropoxy-3-vinylaniline 5 (1 equiv; about 0.2 mmol) was added to a rou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com