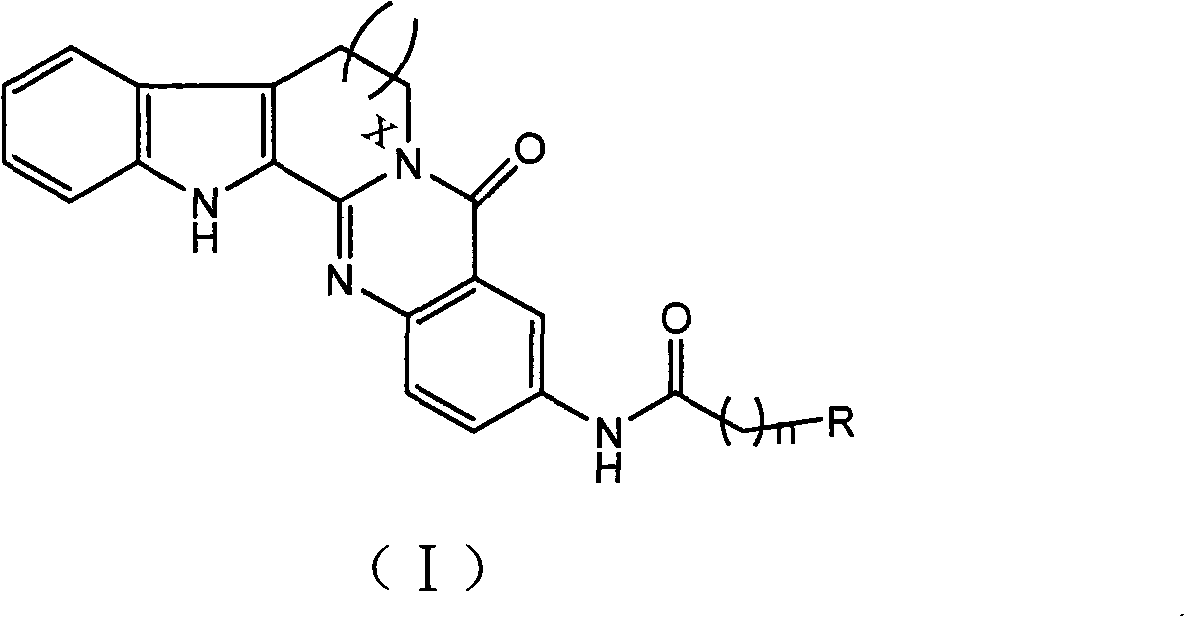
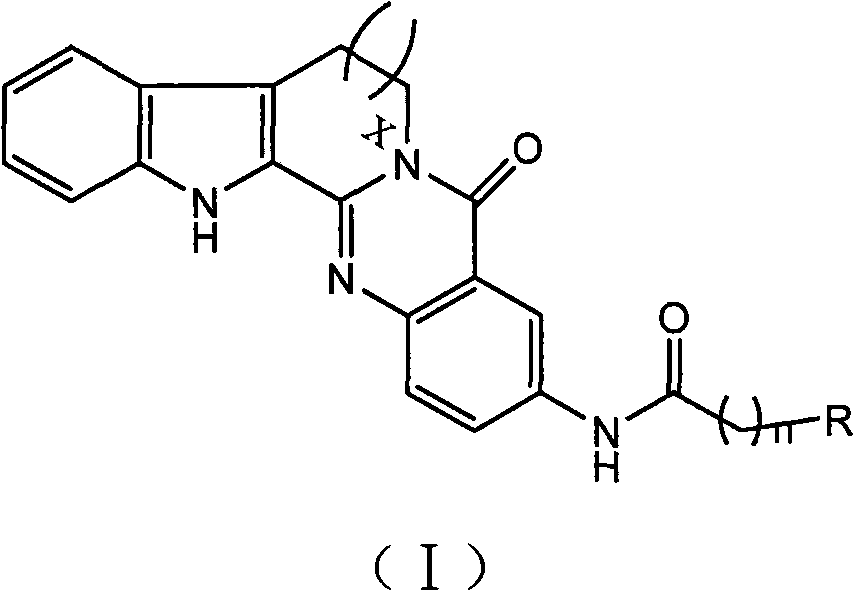
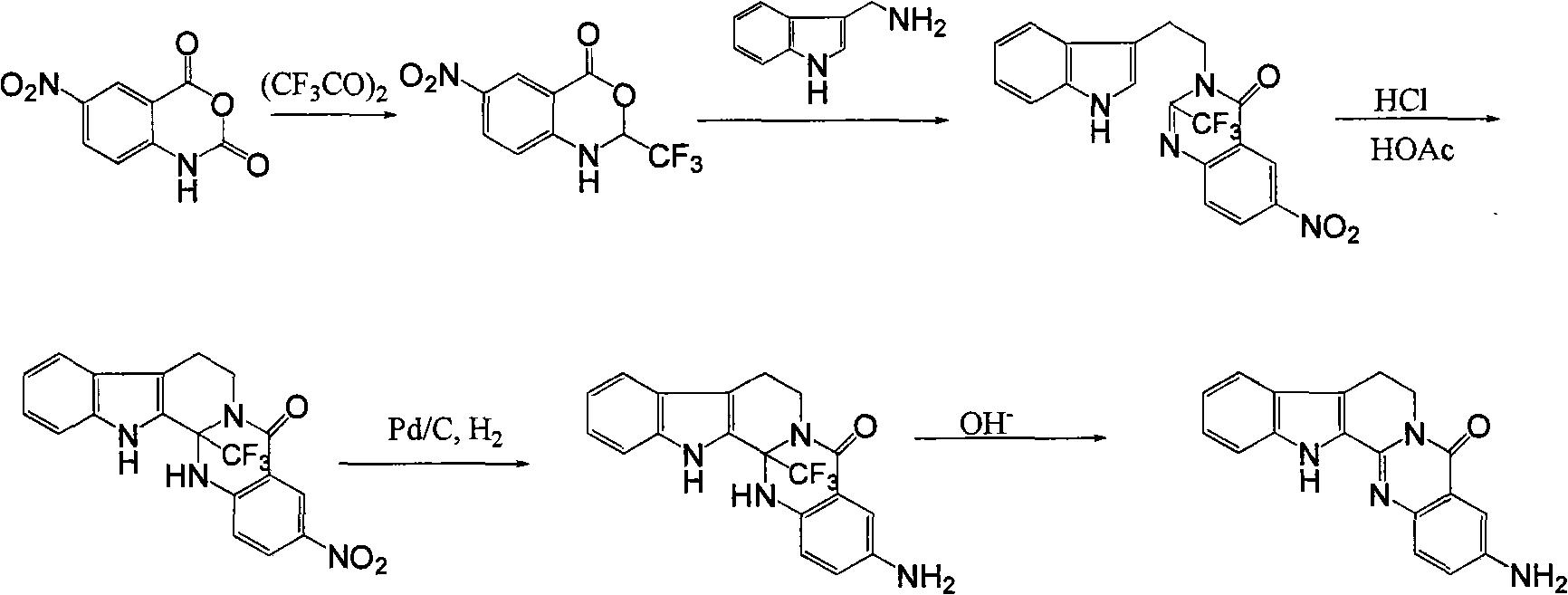
3-aminoalkaneacylamino-rutaecarpine and 3-aminoalkaneacylamino-7,8-dehydrorutaecarpine derivative
An aminoalkanoylamide group and evodiamine technology, which can be used in drug combinations, organic active ingredients, muscular system diseases, etc., can solve the problem of no significant improvement in butyrylcholinesterase activity, and achieve strong inhibitory activity, high selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Synthesis of 3-(2-chloroacetamido)-evodiamine
[0027] Add 1.5g 3-amino-evodiamine and 0.83g K in 90mL dichloromethane 2 CO 3 , add 5 mL of methylene chloride containing 0.48 mL of chloroacetyl chloride dropwise at room temperature, heat to reflux for 4 hours after the dropwise addition, cool, filter, wash with methylene chloride and water successively, and obtain 1.5 g of a green solid after drying. 77%. mp 281.6-284.3°C; 1 H-NMR (400MHz, DMSO-d 6 ): δ11.86(s, 1H), 10.67(s, 1H), 8.49(d, J=2.1, 1H), 7.98(dd, J=8.8, 2.3, 1H), 7.67(dd, J=16.1, 8.4, 2H), 7.48 (d, J=8.3, 1H), 7.26 (t, J=7.7, 1H), 7.09 (t, J=7.5, 1H), 4.46 (t, J=6.8, 2H), 4.32 (s, 2H), 3.18 (t, J=6.8, 2H); ESI-MS: m / z 379 [M+1] + .
[0028] The structural formula of synthetic compound 1 is as follows:
[0029]
Embodiment 2
[0030] Example 2 Synthesis of 3-(2-chloropropionamido)-evodiamine
[0031] In 90mL of dichloromethane, add 1.5g 1.5g 3-amino-evodiamine and 0.83gK 2 CO 3 , add 5 mL of dichloromethane containing 0.53 mL of 3-chloropropionyl chloride dropwise at room temperature, heat to reflux for 4 hours after the dropwise addition, cool, filter, wash with dichloromethane and water successively, and dry to obtain 1.8 g of green solid, producing Rate 90%. mp 275.4-277.0°C; 1 H-NMR (400MHz, DMSO-d 6 ): δ11.84 (s, 1H), 10.44 (s, 1H), 8.51 (d, J=2.3, 1H), 8.00 (dd, J=8.8, 2.4, 1H), 7.66 (dd, J=14.1, 8.4, 2H), 7.49 (d, J=8.2, 1H), 7.26 (t, J=7.5, 1H), 7.09 (t, J=7.4, 1H), 4.46 (t, J=6.8, 2H), 3.93 (t, J = 6.2, 2H), 3.17 (t, J = 6.8, 2H), 2.89 (t, J = 6.2, 2H); ESI-MS: m / z 393 [M+1] + .
[0032] The structural formula of synthetic compound 2 is as follows:
[0033]
Embodiment 3
[0034] Example 3 Synthesis of 3-(2-acetylamino)-7,8-dehydroevodiamine
[0035] Add 0.76g 3-(2-chloroacetamido)-evodiamine to 50mL of dioxane, heat and stir; dissolve 0.54g DDQ in 5mL of dioxane, and slowly add it dropwise to the reaction solution; After the dropwise addition, heat and reflux for 4 hours, collect the precipitate by filtration, treat the precipitate with KOH solution (1.5g KOH dissolved in 25mL water) several times until all DDQ-2H is removed, and dry the obtained solid in vacuum to obtain 0.39g of light brown solid , yield 52%. mp299.6-302.4℃; 1 H-NMR (400MHz, DMSO-d 6 ): δ12.68(s, 1H), 10.69(s, 1H), 8.71(s, 1H), 8.61(d, J=7.4, 1H), 8.16(d, J=7.9, 1H), 8.07(d , J=8.8, 1H), 7.84 (dd, J=14.0, 8.3, 2H), 7.69 (d, J=8.2, 1H), 7.49 (t, J=7.5, 1H), 7.29 (t, J=7.4 , 1H), 4.34(s, 2H); ESI-MS: m / z 377[M+1] + .
[0036] The structural formula of synthetic compound 3 is as follows:
[0037]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com