Application of penehyclidine hydrochloride oral cavity partial drug administration as dental surgery medicine
A technology of penehyclidine hydrochloride and topical administration, which is applied in the application field of dental surgery drugs, and can solve problems such as patient distress and inconvenient treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
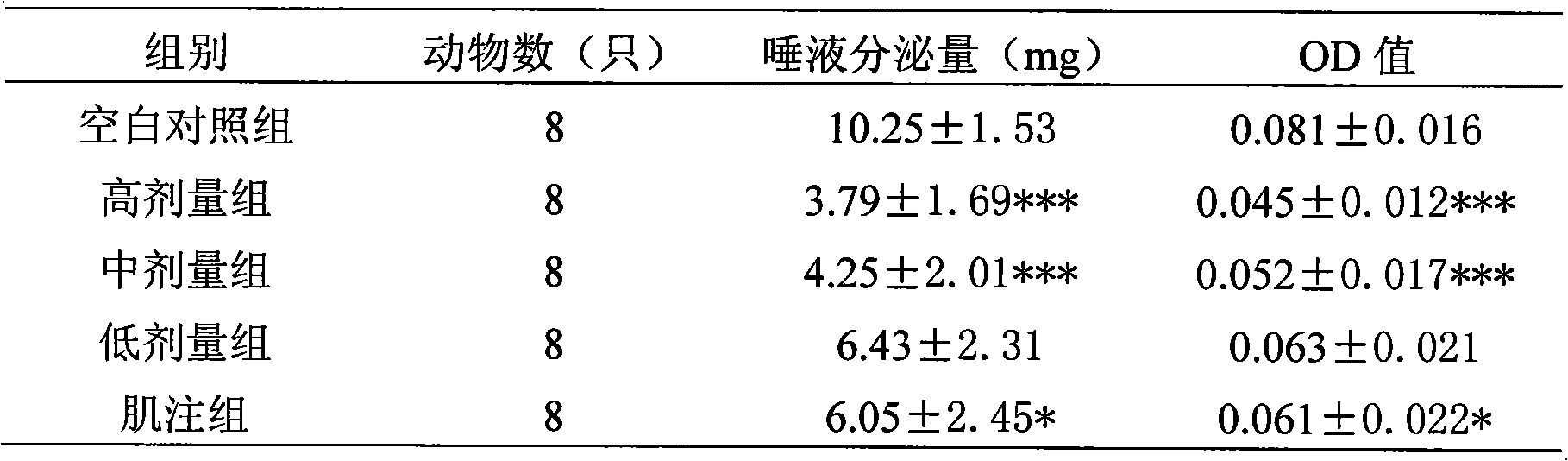
[0007] Embodiment 1 Pharmacodynamics test
[0008] 1. Test material
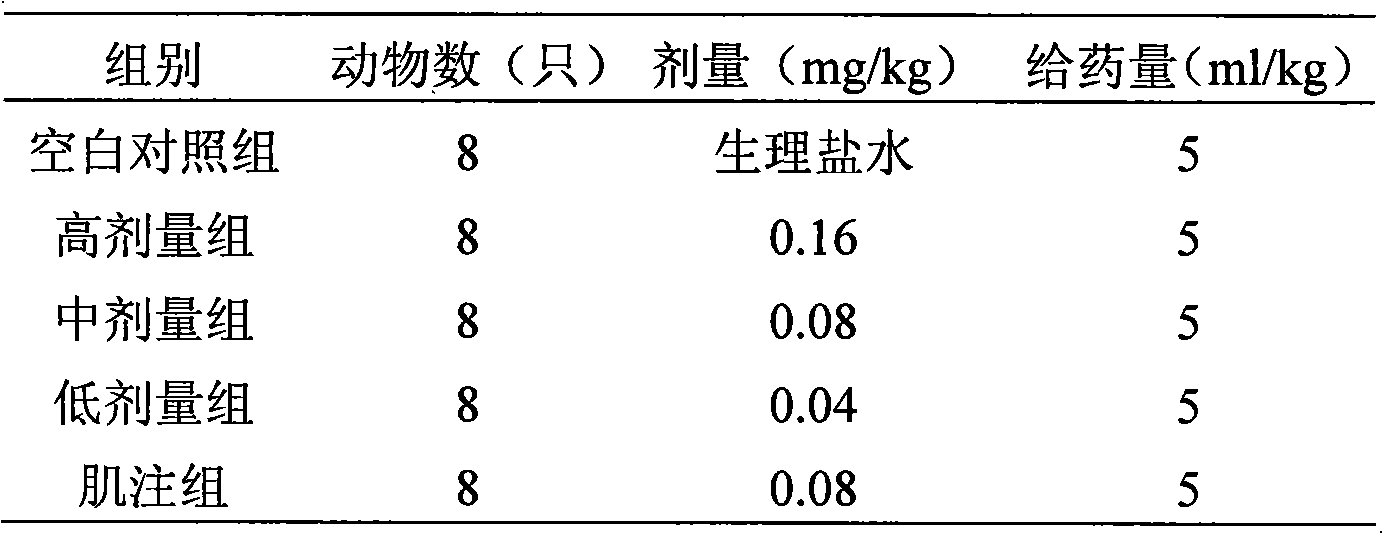
[0009] 1.1 Test drug: Penehyclidine hydrochloride, prepared into 8 μg / ml, 16 μg / ml, 32 μg / ml colorless transparent liquid with sterile water for injection, batch number: 090601, provided by Chengdu Lisite Pharmaceutical Co., Ltd.
[0010] 1.2 Positive control drug: penehyclidine hydrochloride injection, specification: 1ml: 1mg, batch number: 081101, prepared into a 16 μg / ml solution with sterile water for injection, provided by Chengdu Lisite Pharmaceutical Co., Ltd.
[0011] 1.3 Main reagents: phenol red, sodium bicarbonate, and normal saline are commercially available.
[0012] 1.4 Experimental animals Class ICR mice, weighing 18-22 g, half male and half male, were provided by Jiancheng Bill Animal Farm in Jianyang City.
[0013] 1.5 Main instruments Electronic balance, BASIC semi-automatic biochemical analyzer.
[0014] 1.6 Experimental environment The room temperature is 18-24°C and the humidity is 40...
Embodiment 2
[0028] Embodiment 2 Adopt the spray preparation method of oral cavity topical administration mode
[0029] Take an appropriate amount of penehyclidine hydrochloride, add appropriate amount of water to make a solution containing 0.01-10 mg per 1 ml, and put it into a bottle with a spray device.
[0030] A Take 4g of penehyclidine hydrochloride, add 1000ml of water and stir to dissolve, add 1.5g of sodium benzoate, stir and dissolve, then divide into spray bottles, 1ml per bottle.
[0031] B. Add 2 g of penehyclidine hydrochloride, add 1000 ml of water and stir to dissolve, add 1.5 g of sodium benzoate, stir and dissolve, then divide into spray bottles, 1 ml per bottle.
Embodiment 3
[0032] Embodiment 3 Adopt the solution preparation method of local oral administration mode
[0033] Take an appropriate amount of penehyclidine hydrochloride, add appropriate amount of water to make a solution containing 0.01-10 mg per 1 ml, put it into a plastic bottle or a glass bottle, and obtain the product.
[0034] A: Take 1 g of penehyclidine hydrochloride, add 1000 ml of water and stir to dissolve, stir and mix well to dissolve, then pack into vials, 1 ml per bottle, sterilize at 121°C for 10 minutes, and then pack.
[0035] B: Take 2 g of penehyclidine hydrochloride, add 1000 ml of water and stir to dissolve, stir and mix well to dissolve, then dispense into vials, 1 ml per bottle, sterilize at 121°C for 10 minutes, and dispense to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com