Method for preparing acyclovir 2/3 hydrate
一种水合物、化合物的技术,应用在2/3水合物的制备领域,能够解决未见文献报道ACV2/3水合物制备方法和XRD表征数据等问题,达到良好晶型稳定性、晶型保持不变、制备工艺简单的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
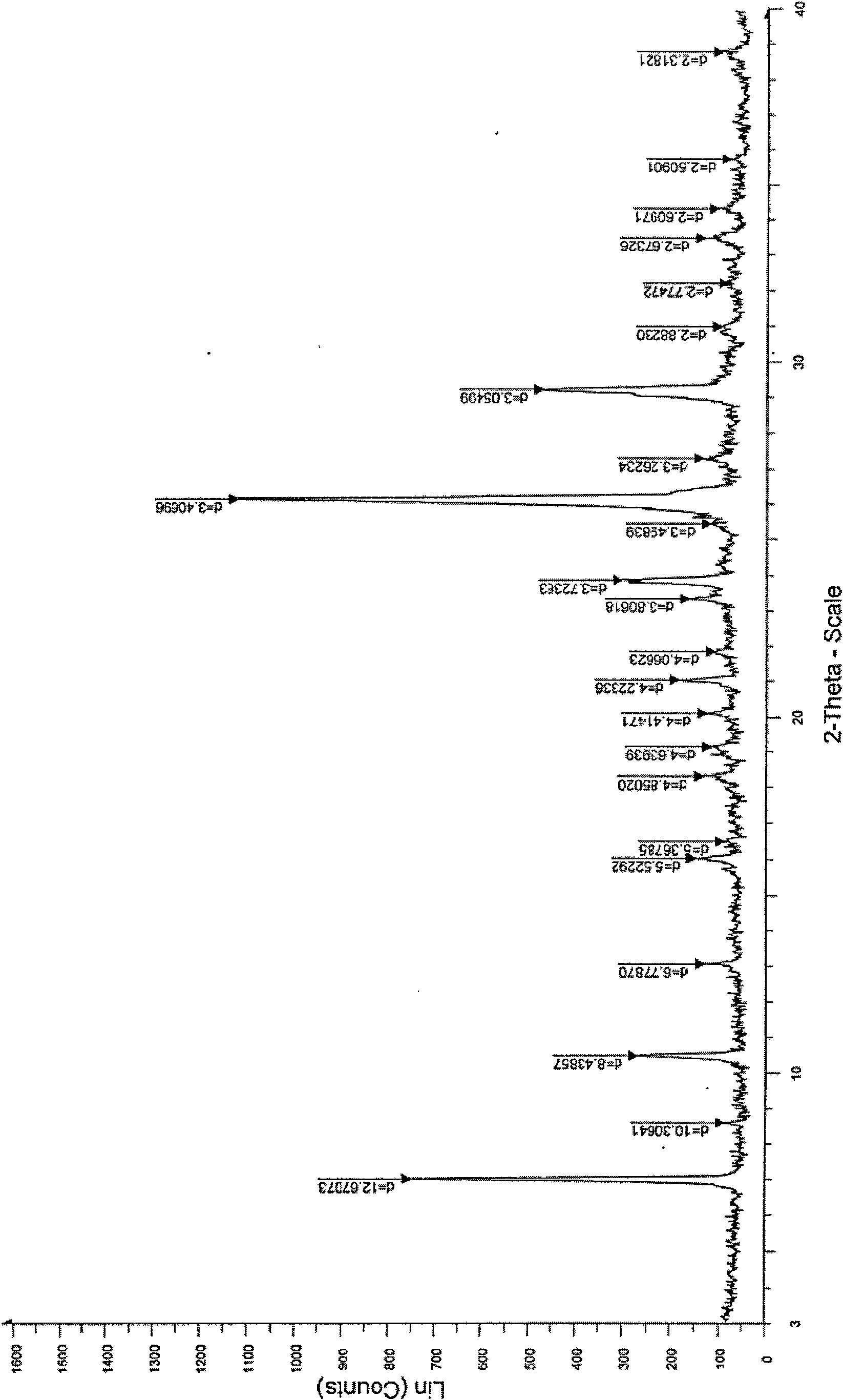
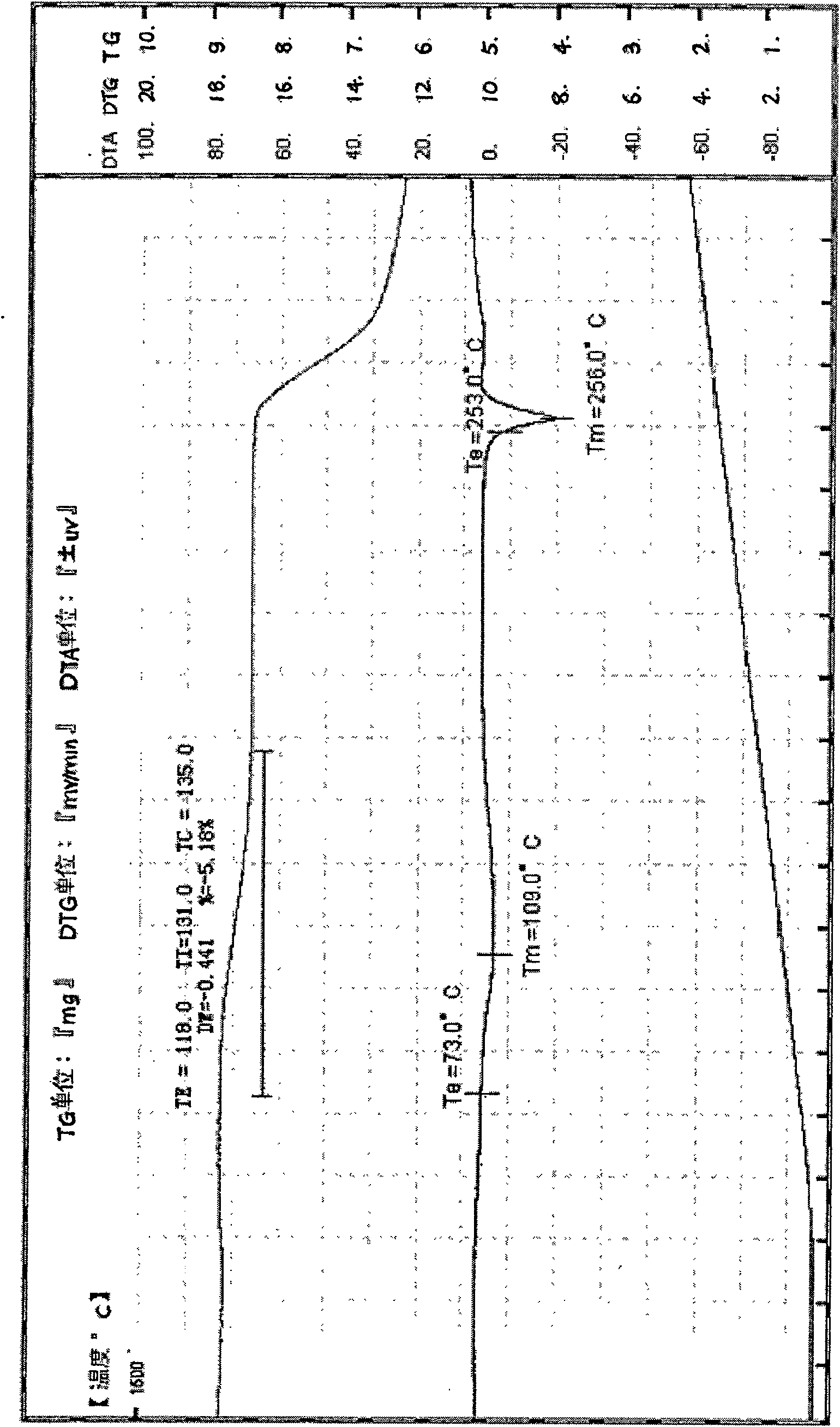
[0027] Heat the mixture of 10g crude acyclovir and 120ml water to 95°C, stir to dissolve, filter, cool the filtrate to 20-25°C, crystallize for 5 hours, filter, and dry the obtained solid in vacuum at 50°C for 20 hours to obtain aciclovir 9.8 g of Lowe crystals were determined by HPLC, and the content was 99.6%. Grind into a powder with a particle size of 200 mesh. Cu-Kα radiation, X-ray powder diffraction represented by 2θ angle and interplanar spacing (d value) at about 7.0 (12.7), about 10.5 (8.4), about 13.1 (6.8), about 16.1 (5.5), about 18.3 There are obvious characteristic absorption peaks at about 21.0 (4.2), about 24.9 (3.7), about 26.2 (3.4), and about 29.2 (3.0). Thermogravimetry (TG) has a weight loss of about 5.1%. Infrared absorption spectrum (KBr tablet) at about 3522, about 3471, about 3438, about 3294, about 3180cm -1 , about 2854cm -1 , about 2698, about 1716cm -1 , about 1631cm -1 , about 1610cm -1 , about 1483cm -1 , about 1388cm -1 , about 1182cm ...
Embodiment 2
[0029] Heat the mixture of 10g crude acyclovir and 150ml water to 90°C, stir to dissolve, filter, cool the filtrate to 20-25°C, crystallize for 5 hours, filter, and dry the obtained solid in vacuum at 50°C for 20 hours to obtain aciclovir 9.6 g of Lowe crystals were determined by HPLC, and the content was 99.7%. Grind into a powder with a particle size of 200 mesh. Cu-Kα radiation, X-ray powder diffraction represented by 2θ angle and interplanar spacing (d value) at about 7.0 (12.7), about 10.5 (8.4), about 13.1 (6.8), about 16.1 (5.5), about 18.3 There are obvious characteristic absorption peaks at about 21.0 (4.2), about 24.9 (3.7), about 26.2 (3.4), and about 29.2 (3.0). Thermogravimetry (TG) has a weight loss of about 5.1%. Infrared absorption spectrum (KBr tablet) at about 3522, about 3471, about 3438, about 3294, about 3180cm -1 , about 2854cm -1 , about 2698, about 1716cm -1 , about 1631cm -1 , about 1610cm -1 , about 1483cm -1 , about 1388cm -1 , about 1182cm ...
Embodiment 3
[0031] Heat the mixture of 10g crude acyclovir and 200ml water to 90°C, stir to dissolve, filter, cool the filtrate to 20-25°C, crystallize for 10 hours, filter, and dry the obtained solid in vacuum at 50°C for 20 hours to obtain aciclovir 9.5 g of Lowe crystals were determined by HPLC, and the content was 99.7%. Grind into a powder with a particle size of 200 mesh. Cu-Kα radiation, X-ray powder diffraction represented by 2θ angle and interplanar spacing (d value) at about 7.0 (12.7), about 10.5 (8.4), about 13.1 (6.8), about 16.1 (5.5), about 18.3 There are obvious characteristic absorption peaks at about 21.0 (4.2), about 24.9 (3.7), about 26.2 (3.4), and about 29.2 (3.0). Thermogravimetry (TG) has a weight loss of about 5.1%. Infrared absorption spectrum (KBr tablet) at about 3522, about 3471, about 3438, about 3294, about 3180cm -1 , about 2854cm -1 , about 2698, about 1716cm -1 , about 1631cm -1 , about 1610cm -1 , about 1483cm -1 , about 1388cm -1 , about 1182cm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com