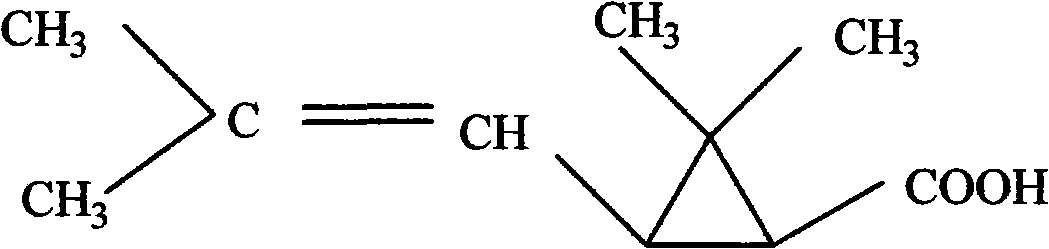
Method for manufacturing rich reverse type first chrysanthemic acid
A manufacturing method and technology of chrysanthemic acid, which is applied in the field of producing the first trans-rich chrysanthemic acid by melting crystallization method, can solve problems such as differences in insecticidal efficacy, and achieve the effects of low production cost, high yield and clean process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
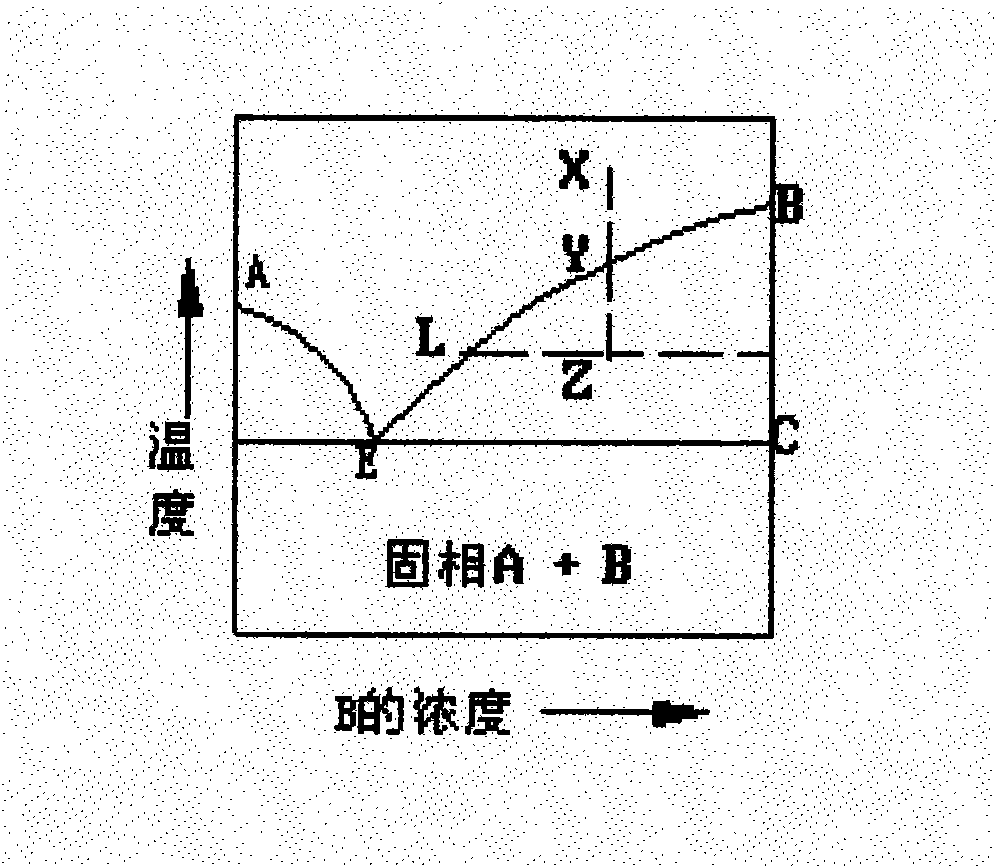
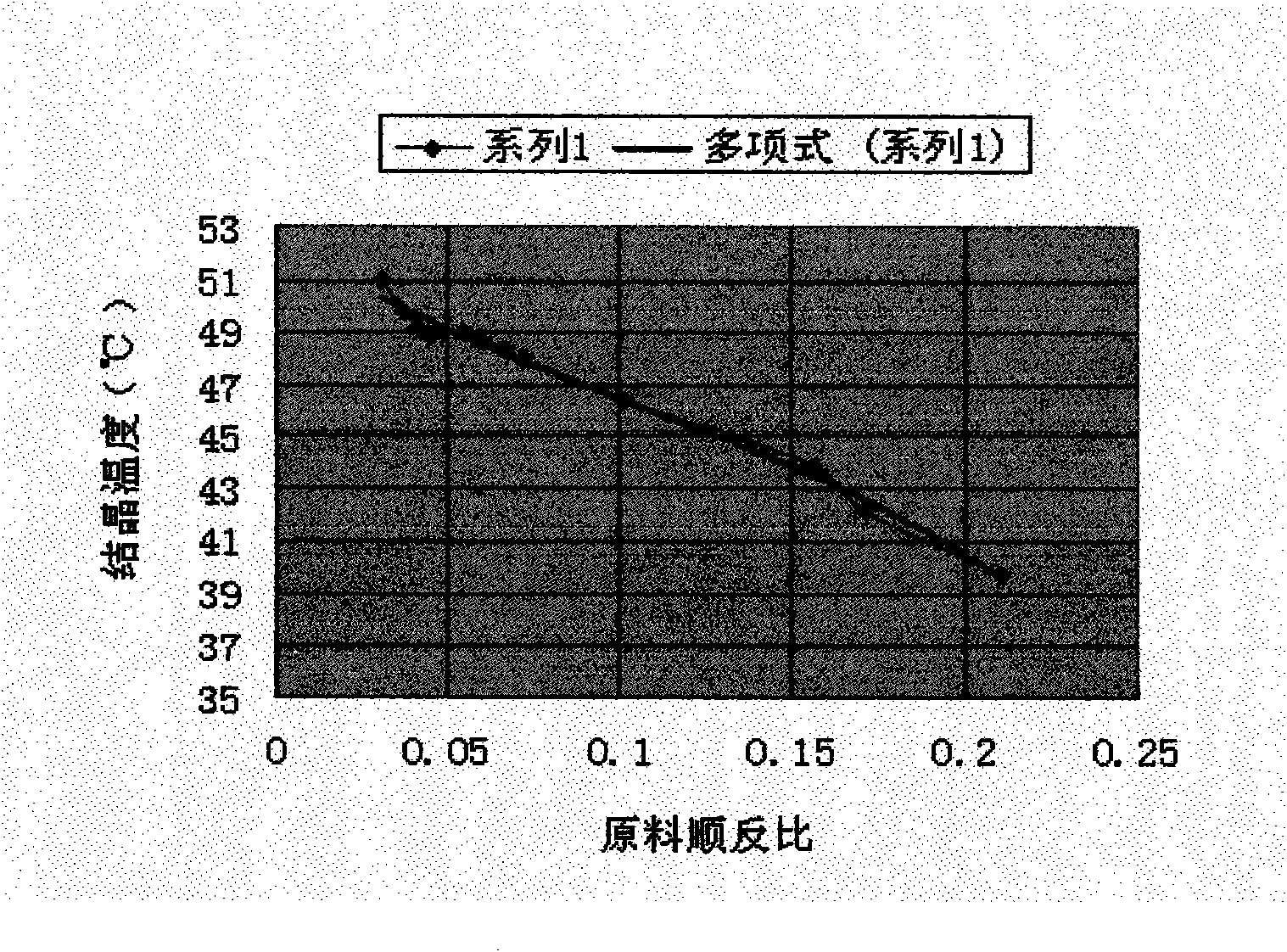
[0022] Accurately weigh the first racemic (±) chrysanthemic acid, melt it completely at 60°C, stir and crystallize slowly at the corresponding crystallization temperature for 2 to 7 hours, and keep warm and filter, then heat up the jacket of the filter tank to make the crystal sweat, and put it while it is hot The liquid is extruded, the temperature is lowered and the crystal is transferred to obtain the first chrysanthemic acid rich in trans with a high cis-to-trans ratio, and the filtrate can be further melted and crystallized.
[0023]
[0024]
[0025]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com