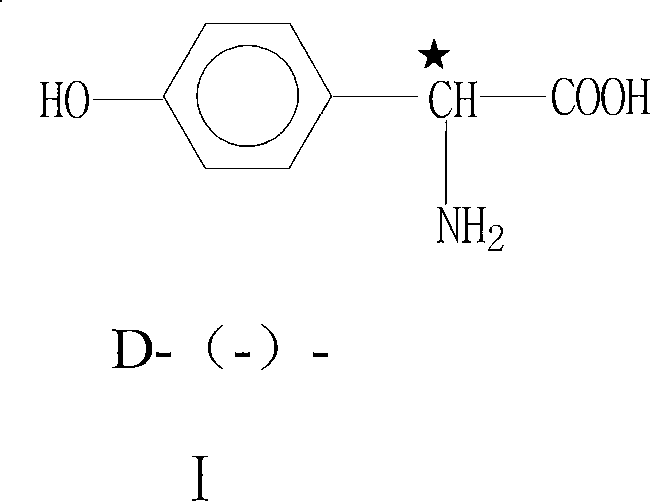
Technique for producing D-(-)-p-hydroxyphenylglycine by aqueous-phase resolution method
A technology of p-hydroxyphenylglycine and water phase is applied in the technical field of producing D-(-)-p-hydroxyphenylglycine by an aqueous phase splitting method, and can solve the problems of high cost, large degree of operation, resource limitation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0031] In a 5000L enamel kettle, put 1600Kg of deionized water, 800Kg of DL-HPG, PTS.H 2 O950Kg was heated up to dissolve, and the temperature of the solution was lowered to 20°C and dried by centrifugation. Get 1520KgDL-HPG·PTS salt
example 2
[0033] Put 1400Kg of deionized water into a 5000L enamel kettle. DL-HPG·PTS470Kg, D-HPG·PTS471Kg, heat until completely dissolved, cool down to 50°C, measure the optical rotation of the solution α=-3°, continue to cool down to 30°C and appear Crystallization, continuously check the optical rotation of the mother liquor, and centrifuge when α=2.5~3. Get D-HPG·PTS200Kg, [α] 20 D =-60°, optical purity 90%, mother liquor optical rotation α=+3°
example 3
[0035] Put the above mother liquor of α=+3° into a dextrorotational separation kettle, 200KgD-HPG·PTS, heat and dissolve and cool down to 50°C to measure the solution α=+3°, continue to cool down to about 30°C to appear crystallization, and constantly check the optical rotation of the mother liquor. When α=-2.5~-3, centrifuge to obtain L-HPG·PTS205Kg, [α] 20 D =+60°, optical purity 90%, mother liquor optical rotation α=-3.1°
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com