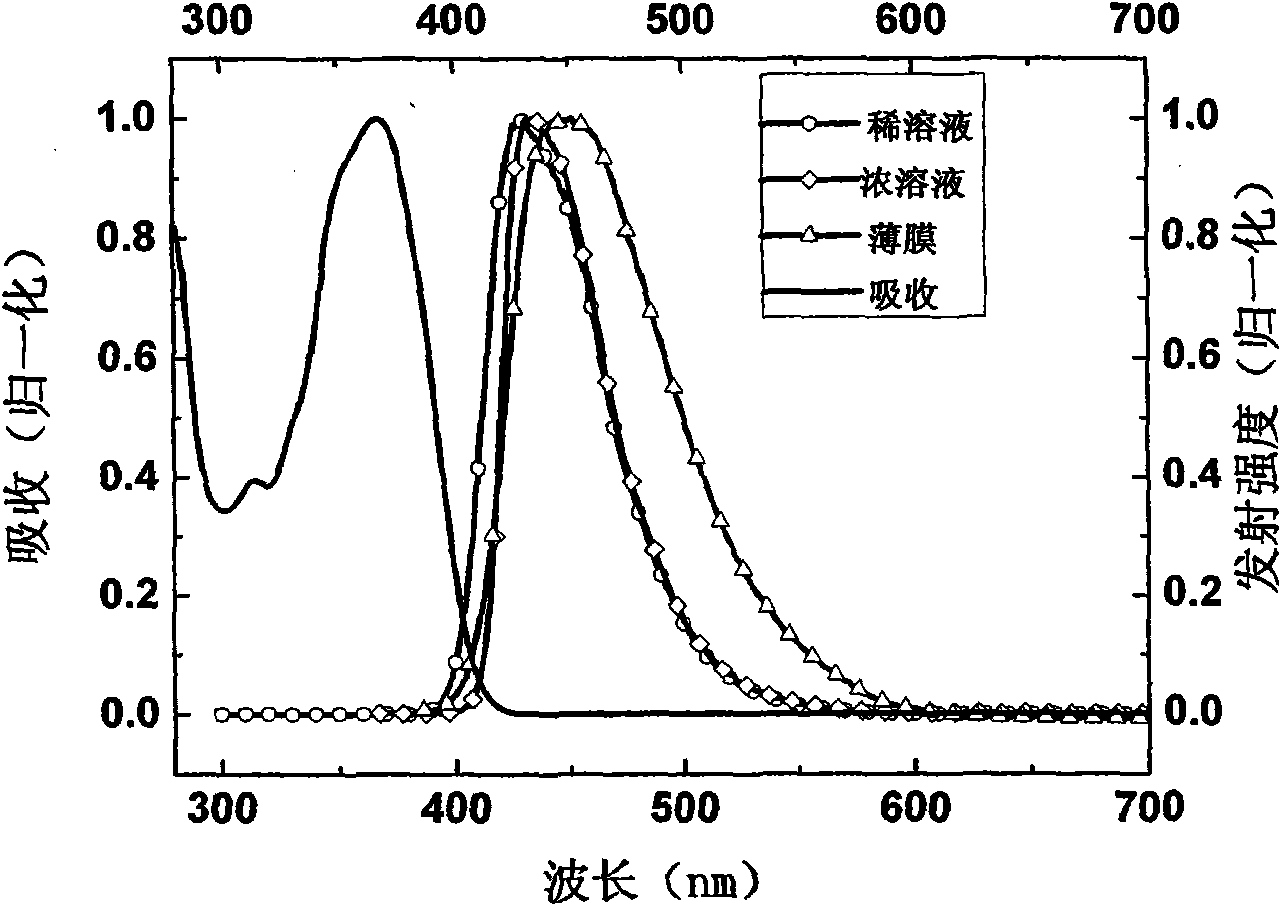
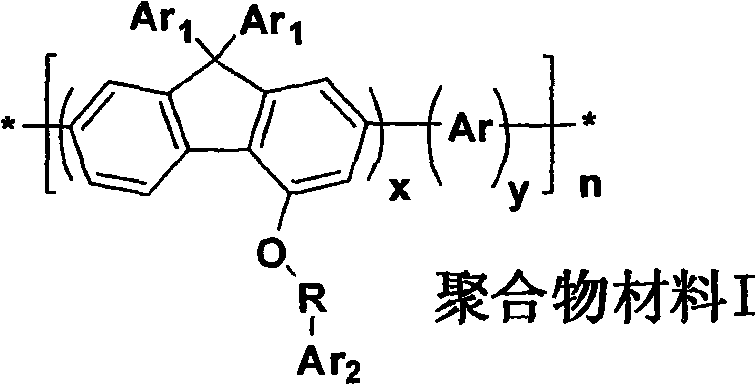
Preparation method and application method of 4-9, 9-diaryl fluorene polymer material containing substituted radical
A polymer material, the technology of diaryl fluorene, which is applied in 9 fields, can solve the problem that the fluorene structural unit is rarely reported, and achieves the effect of mild conditions and simple synthesis steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
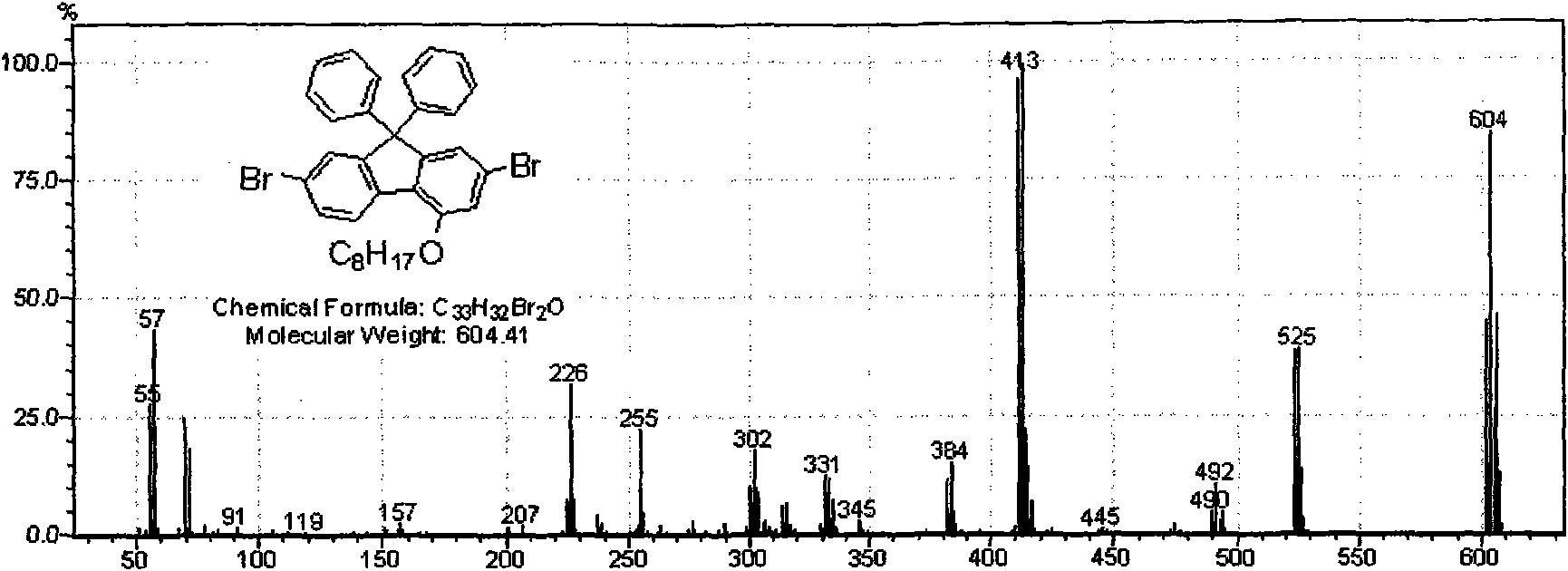
[0042] Embodiment 1, poly(4-octyloxy-9,9-diphenylfluorene)
[0043] Poly(4-(octyloxy)-9, 9-diphenyl-9H-fluorene)
[0044] 6H-3,8-Dibromobenzopyran-6-one
[0045] 3,8-dibromo-6H-benzo[c]chromen-6-one
[0046] Experimental procedure: 2 g (5.92 mmol) of 2,7-dibromofluorenone and 11.83 ml of trifluoroacetic acid were mixed in a two-necked flask. Under the condition of ice-water bath, add Na every 10 minutes 2 CO 4 0.25g, five times in total. Return to room temperature and stir for 48 hours. The reaction was quenched with 40ml of ice water and extracted with dichloromethane (3×30ml). Washed with 10% NaHCO3, dried and rotary evaporated, and purified on a silica gel column with a mixed solvent of petroleum ether:dichloromethane (3:1) to obtain a white solid (yield 70%).
[0047] 2'-(Hydroxydiphenylmethyl)-4,4'-dibromobiphenyl-2-ol
[0048] 4,4'-dibromo-2'-(hydroxydiphenylmethyl)biphenyl-2-ol
[0049] Experimental procedure: get bromo-benzene (15mmol) and magnesium (0.36g, 1...
Embodiment 2
[0059] Example 2, poly-{[2,7-(9,9-spirobifluorene)]-2,7-[4-(octyloxy)-9,9-diphenyl-9H-fluorene]}
[0060] poly-{[2,7-(9,9'-spirobi[fluorene])]-2,7-[4-(octyloxy)-9,9-diphenyl-9H-fluorene]}
[0061] Experimental procedure: Take 2,7-dibromo-4-octyloxy-9,9-diphenylfluorene (0.302g, 0.5mmol, 1equiv) and 9,9-spirobifluorene-2,7-diboronic acid butyl The base ester (0.242g, 0.5mmol, 1equiv) is mixed and dissolved in the mixed solvent of 20ml toluene and tetrahydrofuran, and the catalyst Pd(PPh 3 ) 4 (1-5mol%). Protect from light and pass through nitrogen, then add K 2 CO 3 (1mL, 2mol / L, 2equiv.), react at 90°C for 48 hours, add water after the reaction, use CHCl 3 Extract, dry and concentrate by rotary evaporation, and put into 100mL of methanol. The collected solid was extracted and purified in methanol for 3 days, and reprecipitated in methanol to obtain 0.278 g of a powdery product. GPC: M n = 17 250; PDI = 2.5.
Embodiment 3
[0062] Example 3, poly-{[2,7-(9,9-diphenyl-9H-fluorene)]-2,7-[4-(octyloxy)-9,9-diphenyl-9H- Fluorene]}poly-{[2,7-(9,9-diphenyl-9H-fluorene)]-2,7-[4-(octyloxy)-9,9-diphenyl-9H-fluorene]}
[0063] Experimental procedure: take 2,7-dibromo-4-octyloxy-9,9-diphenylfluorene (0.302g, 0.5mmol, 1equiv) and 9,9-diphenylfluorene-2,7-diboronic acid Butyl ester (0.242g, 0.5mmol, 1equiv) was mixed and dissolved in a mixed solvent of 20ml toluene and tetrahydrofuran, and the catalyst Pd(PPh 3 ) 4 (1-5mol%). Protect from light and pass through nitrogen, then add K 2 CO 3 (1mL, 2mol / L, 2equiv.), react at 90°C for 48 hours, add water after the reaction, use CHCl 3 Extract, dry and concentrate by rotary evaporation, and put into 100mL of methanol. The collected solid was extracted and purified in methanol for 3 days, and reprecipitated in methanol to obtain 0.271 g of a powdery product. GPC: M n = 19 550; PDI = 1.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com